Themed collection Methodology development for protein modifications

Recent advances in the synthesis of C-terminally modified peptides
A go-to compilation of recent strategies to access C-terminally modified peptides contextualized by a discussion of the major synthetic challenges that have historically hampered progress in this area.
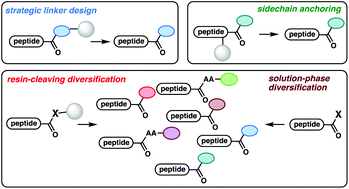
Org. Biomol. Chem., 2020,18, 7253-7272
https://doi.org/10.1039/D0OB01417F
Chemical methods for modification of proteins
The field of protein bioconjugation draws attention from stakeholders in chemistry, biology, and medicine. This review provides an overview of the present status, challenges, and opportunities for organic chemists.

Org. Biomol. Chem., 2020,18, 4669-4691
https://doi.org/10.1039/D0OB00857E
A 2-formylphenylboronic acid (2FPBA)-maleimide crosslinker: a versatile platform for Cys-peptide–hydrazine conjugation and interplay
A heterobifunctional 2-formylphenylboronic acid (2-FPBA)–maleimide crosslinker is explored for the conjugation and interplay between hydrazines, thiols and cysteine peptides.

Org. Biomol. Chem., 2021,19, 6221-6226
https://doi.org/10.1039/D1OB00917F
Clickable gold-nanoparticles as generic probe precursors for facile photoaffinity labeling application
Clickable photoreactive gold nanoparticles have been developed to facilitate one-step preparation of photoaffinity probes for bioactive small molecules and their application to target protein analysis.
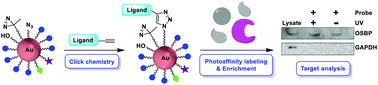
Org. Biomol. Chem., 2021,19, 1268-1273
https://doi.org/10.1039/D0OB01688H
Red-shifted backbone N–H photocaging agents
A 3-nitrodibenzofuran cure provides blue-shifted reactivity in vinylogous photocleavage processes.
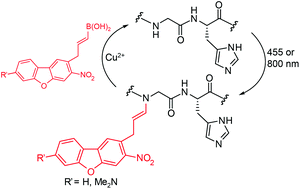
Org. Biomol. Chem., 2020,18, 5110-5114
https://doi.org/10.1039/D0OB00923G
Efficient and selective antibody modification with functionalised divinyltriazines
We have developed a highly efficient disulfide rebridging strategy for the modification of antibodies with substituted divinyltriazine linkers.

Org. Biomol. Chem., 2020,18, 4739-4743
https://doi.org/10.1039/D0OB01002B
Disulphide-mediated site-directed modification of proteins
Site-directed addition of a single thiols handle to proteins by means of temporary disulphide rebridging of solvent exposed disulphides is obtained with a new labelling reagent.
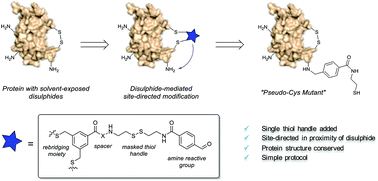
Org. Biomol. Chem., 2020,18, 4717-4722
https://doi.org/10.1039/D0OB00861C
Site-selective modification of proteins using cucurbit[7]uril as supramolecular protection for N-terminal aromatic amino acids
Supramolecular protection of N-terminal aromatic amino acids through complexation with cucurbit[7]uril can enable site-selective protein modification of unfavored motifs.
![Graphical abstract: Site-selective modification of proteins using cucurbit[7]uril as supramolecular protection for N-terminal aromatic amino acids](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D0OB01004A&imageInfo.ImageIdentifier.Year=2020)
Org. Biomol. Chem., 2020,18, 4371-4375
https://doi.org/10.1039/D0OB01004A
Rapid sodium periodate cleavage of an unnatural amino acid enables unmasking of a highly reactive α-oxo aldehyde for protein bioconjugation
A genetically incorporated ThrK unnatural amino acid can undergo rapid periodate oxidation to reveal a reactive internal α-oxo aldehyde.
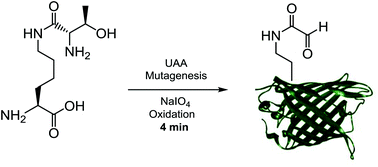
Org. Biomol. Chem., 2020,18, 4000-4003
https://doi.org/10.1039/D0OB00972E
A laccase-catalysed tyrosine click reaction
The tyrosine click reaction of peptides/proteins with the tyrosine modification reagent, N-methyl luminol, was catalysed by a laccase in the presence of molecular oxygen (O2) at 37 °C.
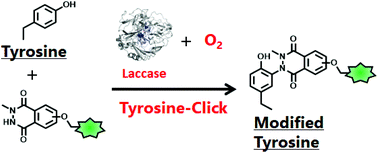
Org. Biomol. Chem., 2020,18, 3664-3668
https://doi.org/10.1039/D0OB00650E
Secondary modification of oxidatively-modified proline N-termini for the construction of complex bioconjugates
A sequential oxidative coupling and oxime or hydrazone ligation method allows construction of bifunctional N-terminal bioconjugates.
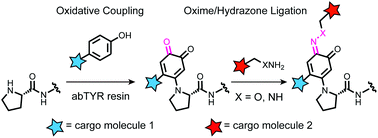
Org. Biomol. Chem., 2020,18, 1881-1885
https://doi.org/10.1039/D0OB00211A
Selective modification of sulfamidate-containing peptides
Hybrid peptides whose N-terminal residues are activated in the form of α-methylisoserine cyclic sulfamidates exhibit rich reactivity as electrophiles, allowing site- and stereoselective modifications at different backbone and side chain positions.
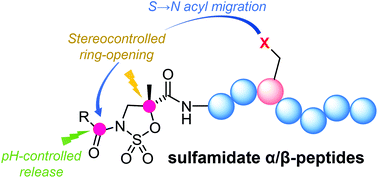
Org. Biomol. Chem., 2020,18, 6265-6275
https://doi.org/10.1039/D0OB01061H
Development of oxetane modified building blocks for peptide synthesis
The synthesis and use of oxetane modified dipeptide building blocks in solution and solid-phase peptide synthesis (SPPS) is reported.
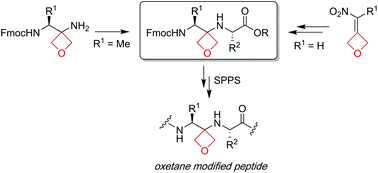
Org. Biomol. Chem., 2020,18, 5400-5405
https://doi.org/10.1039/D0OB01208D
General dual functionalisation of biomacromolecules via a cysteine bridging strategy
A general method that facilitates the modular dual functionalisation of a range of peptides and proteins is reported.
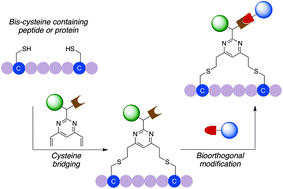
Org. Biomol. Chem., 2020,18, 4224-4230
https://doi.org/10.1039/D0OB00907E
Mutational biosynthesis to generate novel analogs of nosiheptide featuring a fluorinated indolic acid moiety
The target product 6′-fluoro-nosiheptide (6′-F-NOS), along with 6′-fluoro-nosiheptide intermediate (NOS 6′-F-NOSint), was obtained by mutational biosynthesis via 6′-fluoro-MIA feeding into mutant NosL.
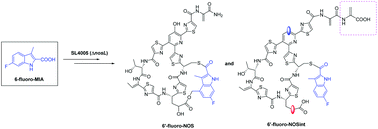
Org. Biomol. Chem., 2020,18, 4051-4055
https://doi.org/10.1039/D0OB00084A
Design, synthesis and structure of a frustrated benzoxaborole and its applications in the complexation of amines, amino acids, and protein modification
Design and synthesis of arylboronic acid 2, the first example of a permanently open “frustrated” benzoxaborole, is described along with an exploration of its application in the complexation of amines and amino acids, and protein modification.

Org. Biomol. Chem., 2020,18, 3492-3500
https://doi.org/10.1039/D0OB00572J
Site-selective protein modification via disulfide rebridging for fast tetrazine/trans-cyclooctene bioconjugation
Site-selective incorporation of a reactive tetrazine tag into therapeutically relevant peptides and proteins via disulfide rebridging allows fast preparation of stable bioconjugates “on-demand”.
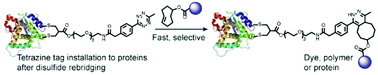
Org. Biomol. Chem., 2020,18, 1140-1147
https://doi.org/10.1039/C9OB02687H
About this collection
The chemical modification of proteins is an important and ever growing field. This collection, guest edited by Professors Annemieke Madder (Ghent University, Belgium), Gonçalo Bernardes (University of Cambridge, UK), and Hiroyuki Nakamura (Tokyo institute of Technology, Japan) showcases the latest advances in Methodology Development for Protein Modifications. The collection scope covers but is not limited to all aspects of new synthetic methods towards protein modification and their application.
New articles will be added to the collection upon publication. Please return to this page frequently to see the collection grow.