Selective modification of sulfamidate-containing peptides†
Abstract
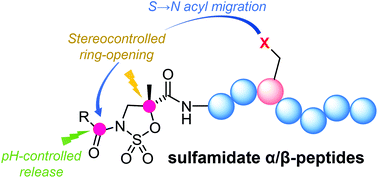
Hybrid peptides whose N-terminal residues are activated in the form of α-methylisoserine-derived cyclic sulfamidates exhibit rich reactivity as electrophiles, allowing site- and stereoselective modifications at different backbone and side chain positions. The unique properties of this scaffold allow the stereocontrolled late-stage functionalization of the peptide backbone by nucleophilic ring opening with fluorescent probes, thiocarbohydrates and tags for strain-promoted azide–alkyne cycloaddition as well as by installing labile N-terminal affinity tags (biotin) and cytotoxic drugs (chlorambucil) for pH-controlled release. Finally, an unexpected base-promoted acyl group migration from the sulfamidate N-terminus allows fast and quantitative intramolecular modification of nucleophilic side chains on the fully unprotected peptides.
- This article is part of the themed collection: Methodology development for protein modifications


 Please wait while we load your content...
Please wait while we load your content...