Development of oxetane modified building blocks for peptide synthesis†
Abstract
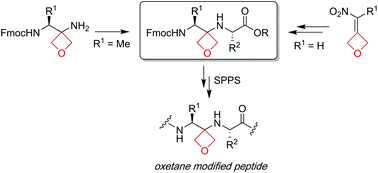
The synthesis and use of oxetane modified dipeptide building blocks in solution and solid-phase peptide synthesis (SPPS) is reported. The preparation of building blocks containing non-glycine residues at the N-terminus in a stereochemically controlled manner is challenging. Here, a practical 4-step route to such building blocks is demonstrated, through the synthesis of dipeptides containing contiguous alanine residues. The incorporation of these new derivatives at specific sites along the backbone of an alanine-rich peptide sequence containing eighteen amino acids is demonstrated via solid-phase peptide synthesis. Additionally, new methods to enable the incorporation of all 20 of the proteinogenic amino acids into such dipeptide building blocks are reported through modifications of the synthetic route (for Cys and Met) and by changes to the protecting group strategy (for His, Ser and Thr).
- This article is part of the themed collections: Methodology development for protein modifications and Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...