Themed collection Editor’s Choice: Main group reagents and catalysts in organic reactions

Base-promoted domino-borylation-protodeboronation strategy
Abundant multi-borylated compounds, such as alkyl 1,2-bis(boronates), gem-diborylalkanes and 1,1,2-tris(boronates) are constructed via the base-promoted DBP strategies, which can undergo selective protodeboronation, giving many borylated molecules.
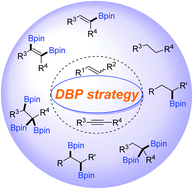
Chem. Commun., 2020,56, 6469-6479
https://doi.org/10.1039/D0CC00614A
Polar organometallic strategies for regioselective C–H metallation of N-heterocyclic carbenes
This Feature Article focuses on the recent emergence of s-block metal-mediated N-heterocyclic carbene metallations and the new opportunities this methodology offers to access unique anionic NHC fragments.
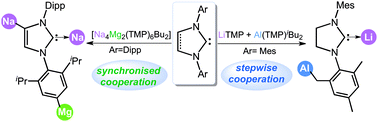
Chem. Commun., 2018,54, 2455-2462
https://doi.org/10.1039/C8CC00049B
Diboration of 3-substituted propargylic alcohols using a bimetallic catalyst system: access to (Z)-allyl, vinyldiboronates
A Pd/Cu catalyst system facilitates the diboration of unactivated propargylic alcohols with pentafluoroboronic acid and diboron to generate (Z)-allyl, vinyldiboronates.
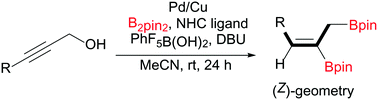
Chem. Commun., 2020,56, 10313-10316
https://doi.org/10.1039/D0CC03563G
Transition-metal-carbene-like intermolecular insertion of a borylene into C–H bonds
In analogy to transition-metal carbene chemistry, [(OC)5Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BN(SiMe3)2] acts as a source of a borylene fragment for selective intermolecular insertion into C–H bonds under very mild conditions.
BN(SiMe3)2] acts as a source of a borylene fragment for selective intermolecular insertion into C–H bonds under very mild conditions.
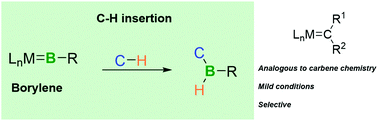
Chem. Commun., 2020,56, 7277-7280
https://doi.org/10.1039/D0CC03075A
Black phosphorus as a metal-free, visible-light-active heterogeneous photoredox catalyst for the direct C–H arylation of heteroarenes
We report for the first time the employment of black phosphorus (BP) as a metal free, visible-light-active and reusable heterogeneous photoredox catalyst for the direct C–H arylation of heteroarenes (furan and thiophene) with aryl diazonium salts.
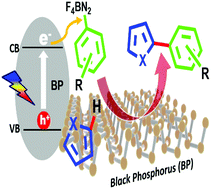
Chem. Commun., 2020,56, 5901-5904
https://doi.org/10.1039/D0CC01874K
Catalyst and additive-free oxidative dual C–H sulfenylation of imidazoheterocycles with elemental sulfur using DMSO as a solvent and an oxidant
A practical, catalyst- and additive-free method for the oxidative dual C–H sulfenylation of imidazoheterocycles with nucleophiles and elemental sulfur using DMSO as both the solvent and oxidant was developed.
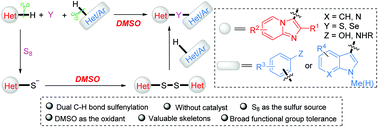
Chem. Commun., 2020,56, 5751-5754
https://doi.org/10.1039/D0CC00043D
Organophotoredox assisted cyanation of bromoarenes via silyl-radical-mediated bromine abstraction
The transition metal-free conversion of aryl bromides into aromatic nitriles using an organophotocatalyst under visible light irradiation.

Chem. Commun., 2020,56, 4240-4243
https://doi.org/10.1039/D0CC00163E
Photocatalytic decarboxylative alkenylation of α-amino and α-hydroxy acid-derived redox active esters by NaI/PPh3 catalysis
Herein, we report the photocatalytic decarboxylative alkenylation reactions of N-(acyloxy)phthalimide derived from α-amino and α-hydroxy acids with 1,1-diarylethene, and with cinnamic acid derivatives through double decarboxylation, using NaI and PPh3 as redox catalysts.
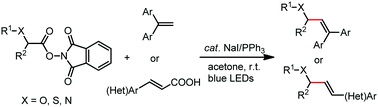
Chem. Commun., 2020,56, 2495-2498
https://doi.org/10.1039/C9CC09654J
Frustrated Lewis pair-catalyzed double hydroarylation of alkynes with N-substituted pyrroles
Metal-free hydroarylation of alkynes with N-substituted pyrroles is shown to be efficiently mediated by B(C6F5)3 to yield variants of dipyrrole methanes. The mechanism is shown to proceed via an FLP addition pathway.
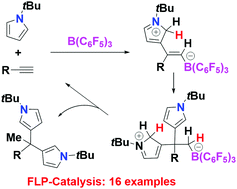
Chem. Commun., 2020,56, 1855-1858
https://doi.org/10.1039/C9CC08654D
Exploration of a KI-catalyzed oxidation system for direct construction of bispyrrolidino[2,3-b]indolines and the total synthesis of (+)-WIN 64821
A facile and environmentally benign KI(cat.)/NaBO3·4H2O oxidation system has been developed for the tandem oxidative aminocyclization/coupling of tryptamines, affording a series of 3a,3a′-bispyrrolidino[2,3-b]indolines with high efficiency (up to 94% yield).
![Graphical abstract: Exploration of a KI-catalyzed oxidation system for direct construction of bispyrrolidino[2,3-b]indolines and the total synthesis of (+)-WIN 64821](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C9CC08646C&imageInfo.ImageIdentifier.Year=2020)
Chem. Commun., 2020,56, 121-124
https://doi.org/10.1039/C9CC08646C
Organoaluminum cations for carbonyl activation
A Lewis acidic cationic organoaluminum catalyst is demonstrated to perform aldehyde dimerization and ketone hydrosilylation via a carbonyl activation pathway.

Chem. Commun., 2019,55, 14629-14632
https://doi.org/10.1039/C9CC08272G
Carbon nitride as a heterogeneous visible-light photocatalyst for the Minisci reaction and coupling to H2 production
Cyanamide functionalised carbon nitride powder is reported as a photocatalyst for direct Minisci-type coupling of heteroarenes with ethers, alcohols, and amides using atmospheric oxygen or a hydrogen evolving co-catalyst as an electron acceptor.
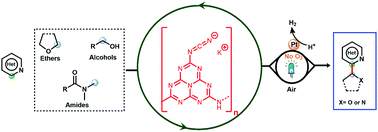
Chem. Commun., 2019,55, 14007-14010
https://doi.org/10.1039/C9CC07348E
Elemental sulfur-promoted one-pot synthesis of 2-(2,2,2-trifluoroethyl)benzoxazoles and their derivatives
An elemental sulfur promoted one-pot synthesis of 2-(2,2,2-trifluoroethyl)benzoxazole derivatives from the reaction of o-aminophenols, thiols, and anilines with 2-bromo-3,3,3-trifluoropropene is reported.
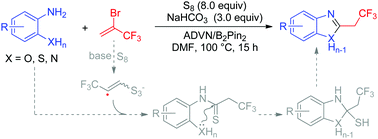
Chem. Commun., 2019,55, 13132-13135
https://doi.org/10.1039/C9CC06822H
Visible light-mediated selective α-functionalization of 1,3-dicarbonyl compounds via disulfide induced aerobic oxidation
A visible light-mediated α-functionalization of 1,3-dicarbonyl compounds with switchable selectivity induced by disulfide is disclosed.
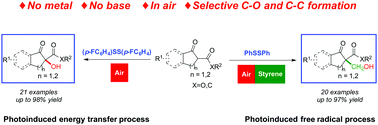
Chem. Commun., 2019,55, 13008-13011
https://doi.org/10.1039/C9CC06544J
1,1-Phosphinoboration of diazomethanes
The reactions of the phosphinoboranes Ph2PBMes2, Ph2PBpin, and Ph2PBcat with the diazomethanes Ph2CN2, C12H8CN2, and EtO2CCHN2 are shown to give products of 1,1-phosphinoboration.
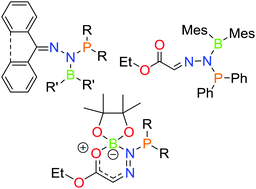
Chem. Commun., 2019,55, 12100-12103
https://doi.org/10.1039/C9CC06914C
Switchable regioselection of C–H thiolation of indoles using different TMS counterions
Simply swapping the counteranions of TMS leads to a switchable regioselectivity in C2– and C3–H thiolation of indoles.
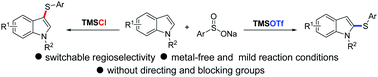
Chem. Commun., 2019,55, 11864-11867
https://doi.org/10.1039/C9CC05652A
Transition-metal-free Intramolecular C–H amination of sulfamate esters and N-alkylsulfamides
The transition-metal-free intramolecular C–H amination of sulfamate esters and N-alkylsulfamides using iodine oxidants, tert-butyl hypoiodite (t-BuOI) and N-iodosuccinimide (NIS) is reported.
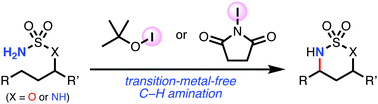
Chem. Commun., 2019,55, 11782-11785
https://doi.org/10.1039/C9CC06410A
Domino N-/C- or N-/N-/C-arylation of imidazoles to yield polyaryl imidazolium salts via atom-economical use of diaryliodonium salts
A Cu-mediated domino di-/triarylation reaction of imidazoles in a single step by using two aryls as well as an anion of a diaryliodonium salt is developed to quickly achieve polyaryl imidazolium salts.

Chem. Commun., 2019,55, 11267-11270
https://doi.org/10.1039/C9CC05237B
A self-catalyzed reaction of 1,2-dibenzoyl-o-carborane with hydrosilanes – formation of new hydrofuranes
FLP-type Si–H bond activation by carbonyls in 1,2-dibenzoyl-o-carborane leading to new hydrofuranes with an o-carboranyl backbone in a self-catalyzed reaction.
Chem. Commun., 2019,55, 10448-10451
https://doi.org/10.1039/C9CC04780H
Thiocyanate radical mediated dehydration of aldoximes with visible light and air
This work describes the first example of dehydration of aldoximes by an in situ generated radical-based hydrogen abstraction reaction.
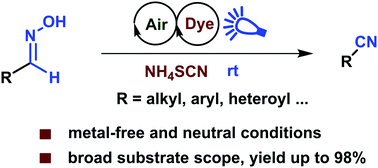
Chem. Commun., 2019,55, 9701-9704
https://doi.org/10.1039/C9CC05354A
P(V) dications: carbon-based Lewis acid initiators for hydrodefluorination
The oxidized P(V) dications [(terpy)(C6Cl4O2)PPh]2+ and [(terpy)(C14H8O2)PPh] are Lewis acidic at the para-carbon of the central ring of the terpy ligand.
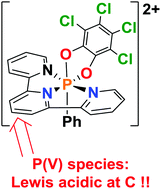
Chem. Commun., 2019,55, 8971-8974
https://doi.org/10.1039/C9CC04010B
Tris(dimethylamino)silylium ion: structure and reactivity of a dimeric silaguanidinium
The formal dimer of an elusive silaguanidinium ion is described. It undergoes spontaneous electrophilic aromatic silylation of electron rich π-systems.
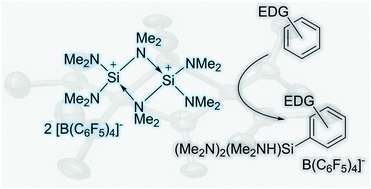
Chem. Commun., 2019,55, 7764-7767
https://doi.org/10.1039/C9CC03625C
Carbonyl and olefin hydrosilylation mediated by an air-stable phosphorus(III) dication under mild conditions
The air-stable Lewis acid [(terpy)PPh][B(C6F5)4]21 mediates the hydrosilylation of aldehydes, ketones, and olefins. The mechanism of these hydrosilylations is considered.
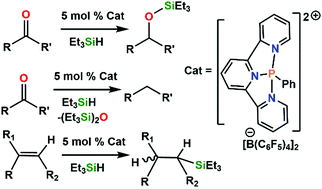
Chem. Commun., 2019,55, 5599-5602
https://doi.org/10.1039/C9CC02460C
Hydroboration without a B–H bond: reactions of the borinium cation [(iPr2N)2B]+ with alkyne, nitrile, ketone and diazomethane
The borinium cation [(iPr2N)2B]+ (1+) is shown to react with PhCCH, PhCN, Ph2CO, and Ph2CN2 to effect a net hydroboration, affording borenium products of 1,1 and 1,2 hydroboration.
![Graphical abstract: Hydroboration without a B–H bond: reactions of the borinium cation [(iPr2N)2B]+ with alkyne, nitrile, ketone and diazomethane](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C9CC01302D&imageInfo.ImageIdentifier.Year=2019)
Chem. Commun., 2019,55, 5155-5158
https://doi.org/10.1039/C9CC01302D
Silylene induced cooperative B–H bond activation and unprecedented aldehyde C–H bond splitting with amidinate ring expansion
Silylene mediated B–H and aldehyde C–H bond splitting were realized under ambient conditions.
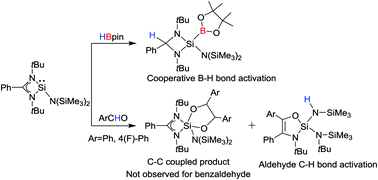
Chem. Commun., 2019,55, 3536-3539
https://doi.org/10.1039/C9CC00296K
Catalyst and additive-free regioselective oxidative C–H thio/selenocyanation of arenes and heteroarenes with elemental sulfur/selenium and TMSCN
Elemental sulfur/selenium and TMSCN act as a novel combined thio/selenocyanation source for direct oxidative C–H thio/selenocyanation of (hetero)arenes under catalyst-free and additive-free conditions.

Chem. Commun., 2018,54, 13367-13370
https://doi.org/10.1039/C8CC07905F
Nitrosonium ion catalysis: aerobic, metal-free cross-dehydrogenative carbon–heteroatom bond formation
Catalytic cross-dehydrogenative coupling of heteroarenes with thiophenols and phenothiazines has been developed under mild and environmentally benign reaction conditions.

Chem. Commun., 2018,54, 13022-13025
https://doi.org/10.1039/C8CC08328B
Oxidative three-component 1,2-alkylarylation of alkenes with alkyl nitriles and N-heteroarenes
Indium-promoted oxidative three-component 1,2-alkylarylation of alkenes with alkyl nitriles and N-heteroarenes is achieved by C(sp3)–H/C(sp2)–H functionalization.
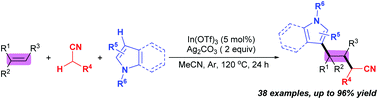
Chem. Commun., 2018,54, 12345-12348
https://doi.org/10.1039/C8CC06509H
Nucleophilic amination of methoxypyridines by a sodium hydride–iodide composite
A new protocol for nucleophilic amination of methoxypyridines and their derivatives was developed using sodium hydride (NaH) in the presence of lithium iodide (LiI).

Chem. Commun., 2018,54, 10324-10327
https://doi.org/10.1039/C8CC05979A
NaI-mediated divergent synthesis of isatins and isoindigoes: a new protocol enabled by an oxidation relay strategy
A new approach for the synthesis of isatins and isoindigoes by an inexpensive and environmentally friendly NaI-mediated transformation is disclosed.
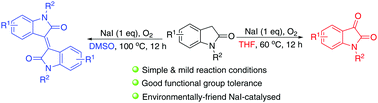
Chem. Commun., 2018,54, 8265-8268
https://doi.org/10.1039/C8CC04471F
Oxidative radical divergent Si-incorporation: facile access to Si-containing heterocycles
Oxidative radical cleavage of Si–H/silyl C(sp3)–H bonds, dual Si–H bonds and Si–H/Si–Si bonds toward Si-heterocycles is presented.
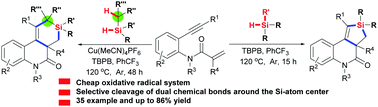
Chem. Commun., 2018,54, 1441-1444
https://doi.org/10.1039/C7CC08964C
A strategy for generating aryl radicals from arylborates through organic photoredox catalysis: photo-Meerwein type arylation of electron-deficient alkenes
Generation of a variety of aryl radicals from arylboronic acids through metal-free photoredox catalysis under mild conditions.
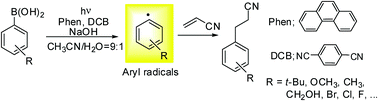
Chem. Commun., 2018,54, 1257-1260
https://doi.org/10.1039/C7CC09140K
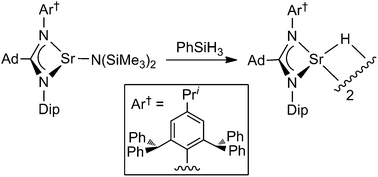
Kinetic stabilisation of a molecular strontium hydride complex using an extremely bulky amidinate ligand
The first dimeric strontium hydride complex (see picture, Dip = 2,6-diisopropylphenyl, Ad = 1-adamantyl), and analogous magnesium hydride systems, have been kinetically stabilised by extremely bulky amidinate ligands, developed for this study.
Chem. Commun., 2018,54, 786-789
https://doi.org/10.1039/C7CC09362D
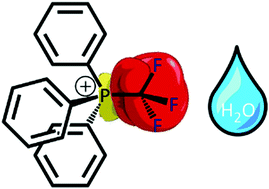
Air- and water-stable Lewis acids: synthesis and reactivity of P-trifluoromethyl electrophilic phosphonium cations
Electrophilic phosphonium cations (EPCs) containing a –CF3 group are stable to air, water, alcohol and strong Brønsted acid and function as Lewis acid catalysts without requiring anhydrous reaction conditions.
Chem. Commun., 2018,54, 662-665
https://doi.org/10.1039/C7CC09128A
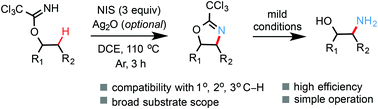
Radical-mediated intramolecular β-C(sp3)–H amidation of alkylimidates: facile synthesis of 1,2-amino alcohols
A new radical-mediated intramolecular β-C(sp3)–H amidation reaction of O-alkyl trichloro- or arylimidates is reported.
Chem. Commun., 2018,54, 515-518
https://doi.org/10.1039/C7CC08897C
About this collection
Collated by ChemComm Chair, Douglas Stephan (University of Toronto, Canada), this collection explores exciting research in the area of main group reagents and catalysts in organic reactions published in the journal. The use of main group species either as stoichiometric reagents or as catalysts is an area that has garnered much attention in the last decade. This area continues to offering new tools for organic synthesis, which are either alternatives or complementary to transition metal catalysts. In this collection, we highlight recent works have that have contributed to this important cutting edge area.