Transition-metal-free Intramolecular C–H amination of sulfamate esters and N-alkylsulfamides†
Abstract
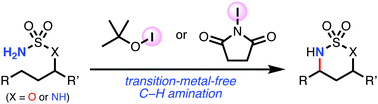
The transition-metal-free intramolecular C–H amination of sulfamate esters using iodine oxidants, tert-butyl hypoiodite (t-BuOI) and N-iodosuccinimide (NIS) is reported. A method using NIS was also successfully applied to the oxidative cyclization of N-alkylsulfamides.
- This article is part of the themed collection: Editor’s Choice: Main group reagents and catalysts in organic reactions


 Please wait while we load your content...
Please wait while we load your content...