Air- and water-stable Lewis acids: synthesis and reactivity of P-trifluoromethyl electrophilic phosphonium cations†
Abstract
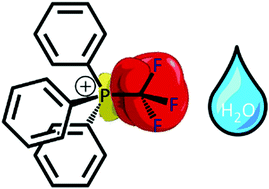
A new class of electrophilic phosphonium cations (EPCs) containing a –CF3 group attached to the phosphorus(V) center is readily accessible in high yields, via a scalable process. These species are stable to air, water, alcohol and strong Brønsted acid, even at raised temperatures. Thus, P–CF3 EPCs are more robust than previously reported EPCs containing P–X moieties (X = F, Cl, OR), and despite their reduced Lewis acidity they function as Lewis acid catalysts without requiring anhydrous reaction conditions.
- This article is part of the themed collection: Editor’s Choice: Main group reagents and catalysts in organic reactions


 Please wait while we load your content...
Please wait while we load your content...