P(v) dications: carbon-based Lewis acid initiators for hydrodefluorination†
Abstract
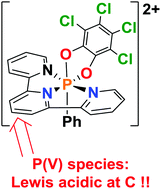
The P(III) dication [(terpy)PPh]2+ is oxidized by reaction with o-chloranil or 9,10-phenanthrenequinone to give the P(V) dications [(terpy)(C6Cl4O2)PPh]2+ and [(terpy)(C14H8O2)PPh]2+, respectively. These species are coordinatively saturated at phosphorus and yet display the ability to effect Lewis acid initiation of hydrodefluorination of fluoroalkanes. Experimental and computational data support the notion that the P(V) dications are Lewis acidic at the para-carbon of the central ring of the terpy ligand.
- This article is part of the themed collection: Editor’s Choice: Main group reagents and catalysts in organic reactions


 Please wait while we load your content...
Please wait while we load your content...