Photocatalytic decarboxylative alkenylation of α-amino and α-hydroxy acid-derived redox active esters by NaI/PPh3 catalysis†
Abstract
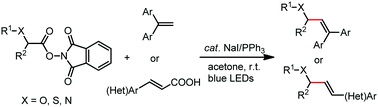
Herein, we report the photocatalytic decarboxylative alkenylation reactions of N-(acyloxy)phthalimide derived from α-amino and α-hydroxy acids with 1,1-diarylethene, and with cinnamic acid derivatives through double decarboxylation, using sodium iodide and triphenylphosphine as redox catalysts. The reaction proceeds under mild irradiation conditions with visible blue light (440 nm or 456 nm) in an acetone solvent without recourse to transition-metal or organic dye based photoredox catalysts. The reaction proceeds via photoactivation of a transiently self-assembled chromophore from N-(acyloxy)phthalimide and NaI/PPh3. Solvation plays a crucial role in the reactivity.
- This article is part of the themed collections: Editor’s Choice: Main group reagents and catalysts in organic reactions and Most popular organic chemistry articles


 Please wait while we load your content...
Please wait while we load your content...