Themed collection Electron delocalization and aromaticity: 150 years of the Kekulé benzene structure

Celebrating the 150th anniversary of the Kekulé benzene structure
This themed issue presents a collection of articles on ‘Electron delocalization and aromaticity: Celebrating the 150th Anniversary of the Kekulé Benzene Structure’.
Phys. Chem. Chem. Phys., 2016,18, 11587-11588
https://doi.org/10.1039/C6CP90088G
Beyond organic chemistry: aromaticity in atomic clusters
We describe joint experimental and theoretical studies carried out collaboratively in the authors' labs for understanding the structures and chemical bonding of novel atomic clusters, which exhibit aromaticity.
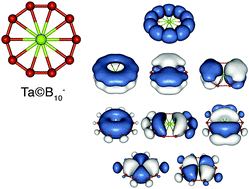
Phys. Chem. Chem. Phys., 2016,18, 11589-11605
https://doi.org/10.1039/C5CP07465G
Aromatic cages B0/+42: unprecedented existence of octagonal holes in boron clusters
The cluster B42+ exhibits an aromatic cage-like structure containing remarkable octagonal holes.

Phys. Chem. Chem. Phys., 2016,18, 11620-11623
https://doi.org/10.1039/C5CP07342A
Bis(triisopropylsilylethynyl)-substituted pyrene-fused tetraazaheptacene: synthesis and properties
The synthesis and characterisation of pyrene-fused tetraazaheptacene that is constituted of two terminal pyrene units and a central tetraazaanthracene core are reported.

Phys. Chem. Chem. Phys., 2016,18, 11616-11619
https://doi.org/10.1039/C5CP07656K
Endohedral Ca@B38: stabilization of a B382− borospherene dianion by metal encapsulation
We present the viability of an endohedral Cs Ca@B38 which contains a Cs B382− borospherene cage composed of interwoven boron double chains with a σ + π double delocalization bonding pattern, extending the Bnq (q = n − 40) borospherene family from n = 39–42 to n = 38.
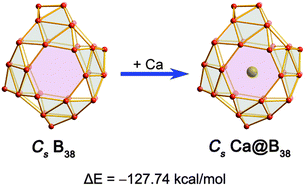
Phys. Chem. Chem. Phys., 2016,18, 11610-11615
https://doi.org/10.1039/C5CP06169E
How does tetraphenylethylene relax from its excited states?
Photocyclization play a key role in the deactivation mechanism of tetraphenylethylene.
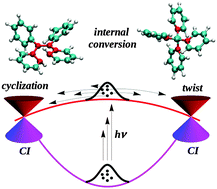
Phys. Chem. Chem. Phys., 2016,18, 11606-11609
https://doi.org/10.1039/C5CP04546K
The degree of π electron delocalization and the formation of 3D-extensible sandwich structures
DFT calculations suggest that the feasible ligands of a 3D sandwich complex should have locally delocalized π electrons.
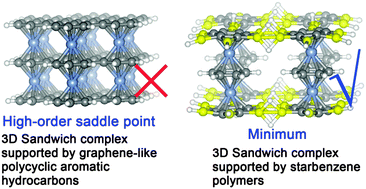
Phys. Chem. Chem. Phys., 2016,18, 11942-11950
https://doi.org/10.1039/C5CP07372C
Aromatic character of planar boron-based clusters revisited by ring current calculations
The planarity of small boron-based clusters is the result of an interplay between geometry, electron delocalization, covalent bonding and stability.
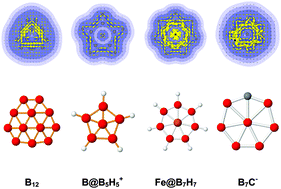
Phys. Chem. Chem. Phys., 2016,18, 11919-11931
https://doi.org/10.1039/C5CP07391J
New insights into aromatic pathways of carbachlorins and carbaporphyrins based on calculations of magnetically induced current densities
The aromatic pathways of carbaporphyrins and carbachlorins that are based on magnetically induced current density DFT-GIMIC calculations are presented and discussed.
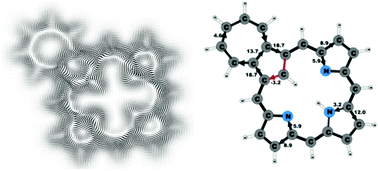
Phys. Chem. Chem. Phys., 2016,18, 11932-11941
https://doi.org/10.1039/C5CP06987D
The stability of biradicaloid versus closed-shell [E(μ-XR)]2 (E = P, As; X = N, P, As) rings. Does aromaticity play a role?
High-level CASSCF calculations, supplemented with MCQDPT ab initio calculations, demonstrate that the [E(μ-XH)]2 (E = P, As; X = N, P, As) compounds possess one planar and one butterfly-like isomers.
![Graphical abstract: The stability of biradicaloid versus closed-shell [E(μ-XR)]2 (E = P, As; X = N, P, As) rings. Does aromaticity play a role?](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C5CP07263H&imageInfo.ImageIdentifier.Year=2016)
Phys. Chem. Chem. Phys., 2016,18, 11879-11884
https://doi.org/10.1039/C5CP07263H
10-π-Electron arenes à la carte: structure and bonding of the [E–(CnHn)–E]n−6 (E = Ca, Sr, Ba; n = 6–8) complexes
Alkaline earth metals (Ca, Sr, and Ba) can form inverted sandwich compounds with C6H6, C7H7+, and C8H82+ of Dnh symmetry holding planar 10-π-electron aromatic cores.
![Graphical abstract: 10-π-Electron arenes à la carte: structure and bonding of the [E–(CnHn)–E]n−6 (E = Ca, Sr, Ba; n = 6–8) complexes](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6CP00671J&imageInfo.ImageIdentifier.Year=2016)
Phys. Chem. Chem. Phys., 2016,18, 11909-11918
https://doi.org/10.1039/C6CP00671J
Edge chlorination of hexa-peri-hexabenzocoronene investigated by density functional theory and vibrational spectroscopy
The molecular structure and vibrational properties of perchlorinated HBC and the parent HBC have been investigated by density functional theory calculations and vibrational spectroscopy.
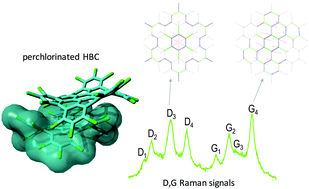
Phys. Chem. Chem. Phys., 2016,18, 11869-11878
https://doi.org/10.1039/C5CP07755A
Heteroleptic Ru(II)-bipyridine complexes based on hexylthioether-, hexyloxy- and hexyl-substituted thienylenevinylenes and their application in dye-sensitized solar cells
A series of eight Ru(II) sensitizers incorporating thienylenevinylene-functionalised bipyridines as ancillary ligand is reported.

Phys. Chem. Chem. Phys., 2016,18, 11901-11908
https://doi.org/10.1039/C5CP07753B
An electronic aromaticity index for large rings
We introduce a new electronic aromaticity index, AV1245, consisting of an average of the 4-center indices along the ring that keep a positional relationship of 1, 2, 4, 5.
Phys. Chem. Chem. Phys., 2016,18, 11839-11846
https://doi.org/10.1039/C6CP00636A
Understanding the molecular switching properties of octaphyrins
Triggering Hückel–Möbius topological and aromaticity switches in octaphyrins by protonation and redox reactions.
Phys. Chem. Chem. Phys., 2016,18, 11885-11900
https://doi.org/10.1039/C5CP07413D
Magnetic resonance energy and topological resonance energy
Magnetic resonance energy (MRE) derived from the ring-current diamagnetic susceptibility was examined in conjunction with topological resonance energy (TRE).

Phys. Chem. Chem. Phys., 2016,18, 11847-11857
https://doi.org/10.1039/C5CP06471F
Electron conjugation versus π–π repulsion in substituted benzenes: why the carbon–nitrogen bond in nitrobenzene is longer than in aniline
Computations show that the enhanced π–π repulsion in nitrobenzene, contributed by both the electrostatic interaction and the Pauli exchange, is responsible for the stretched C–N bond in nitrobenzene (1.486 Å) compared with the C–N bond in aniline (1.407 Å).
Phys. Chem. Chem. Phys., 2016,18, 11821-11828
https://doi.org/10.1039/C6CP00471G
Cyclic π-electron delocalization in non-planar linear acenes
Based on the experimental data of newly determined structures of per-substituted naphthalenes by halogen atoms (F, Cl and Br) and perchloroanthracene, as well as on the molecular modeling we have shown that deviation from planarity leads to relatively small changes in the cyclic π-electron delocalization of acenes.
Phys. Chem. Chem. Phys., 2016,18, 11813-11820
https://doi.org/10.1039/C5CP07056B
Computational study on donor–acceptor optical markers for Alzheimer's disease: a game of charge transfer and electron delocalization
The length of the conjugated double bond chain in DANIR dyes modulates the charge transfer character, non-radiative deactivation pathways and affinity for amyloid-β fibril.
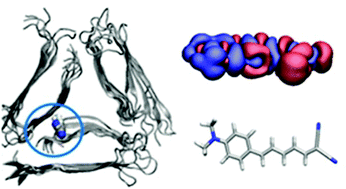
Phys. Chem. Chem. Phys., 2016,18, 11634-11643
https://doi.org/10.1039/C5CP07274C
Understanding conductivity in molecular switches: a real space approach in octaphyrins
In recent years, expanded porphyrins have emerged as a promising class of π-conjugated switches whose conductance is studied from the electron density.
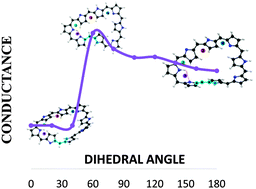
Phys. Chem. Chem. Phys., 2016,18, 11829-11838
https://doi.org/10.1039/C5CP07411H
How amino and nitro substituents direct electrophilic aromatic substitution in benzene: an explanation with Kohn–Sham molecular orbital theory and Voronoi deformation density analysis
Molecular orbitals of aniline explain electrophilic substitution, whereas for nitrobenzene charge rearrangements are needed.
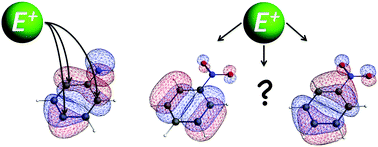
Phys. Chem. Chem. Phys., 2016,18, 11624-11633
https://doi.org/10.1039/C5CP07483E
Electron delocalization and electron density of small polycyclic aromatic hydrocarbons in singlet excited states
Electron delocalization allows us to study the similarity and aromaticity of PAHs in excited states, and can be correlated with the excitation energies.
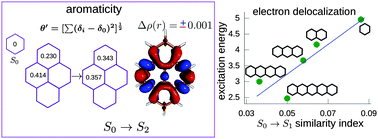
Phys. Chem. Chem. Phys., 2016,18, 11792-11799
https://doi.org/10.1039/C5CP06993A
The making of ring currents
The π-electron diatropic ring current emerging throughout the trimerization of acetylene to benzene.

Phys. Chem. Chem. Phys., 2016,18, 11800-11812
https://doi.org/10.1039/C5CP07250F
[60]Fullerene–porphyrin [n]pseudorotaxanes: self-assembly, photophysics and third-order NLO response
Herein we report a series of porphyrin and methano[60]fullerene that undergo self-assembly.
![Graphical abstract: [60]Fullerene–porphyrin [n]pseudorotaxanes: self-assembly, photophysics and third-order NLO response](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C5CP06055A&imageInfo.ImageIdentifier.Year=2016)
Phys. Chem. Chem. Phys., 2016,18, 11858-11868
https://doi.org/10.1039/C5CP06055A
Decay rate of real space delocalization measures: a comparison between analytical and test systems
We examine in this contribution the possible relation between the spatial decay rate of real space delocalization measures and the insulating- or metallic-like character of molecular and extended systems.
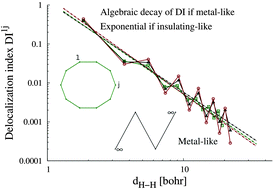
Phys. Chem. Chem. Phys., 2016,18, 11772-11780
https://doi.org/10.1039/C5CP06098B
Aromatic stabilization of functionalized corannulene cations
Conservation of aromaticity of 6-membered rings along with vanishing anti-aromatic character of central 5-membered ring was found to be the main reason for exceptional stability of hub-isomer. Functionalization of corannulene moiety at rim- or spoke-site resulted in dramatic elimination of aromaticity of 6-membered rings thus resulting to dramatic reduce of their stability.
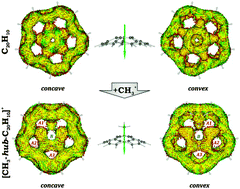
Phys. Chem. Chem. Phys., 2016,18, 11781-11791
https://doi.org/10.1039/C5CP07002C
Perimeter ring currents in benzenoids from Pauling bond orders
Benzenoid perimeter currents within the Randić conjugated-circuit model follow directly from a simple calculation of Pauling bond orders and Kekulé count.

Phys. Chem. Chem. Phys., 2016,18, 11756-11764
https://doi.org/10.1039/C5CP07000G
Topological definition of ring currents
A definition of ring currents in a velocity vector field is proposed according to topological criteria: ring currents are axial vortices confined in, or rotating beyond, a separatrix, i.e., the boundary which marks the limits of the vortex.
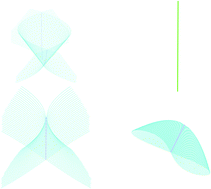
Phys. Chem. Chem. Phys., 2016,18, 11765-11771
https://doi.org/10.1039/C5CP06865G
Can the current density map topology be extracted from the nucleus independent chemical shifts?
It is shown that no unique current density topology can be obtained from (sets of) NICS values. Therefore, the use of NICS indices as aromaticity indices without prior analysis of the current density map is strongly discouraged.
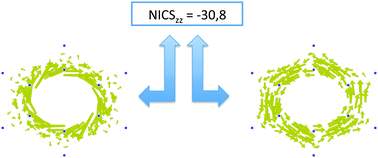
Phys. Chem. Chem. Phys., 2016,18, 11746-11755
https://doi.org/10.1039/C5CP07170D
Viability of aromatic all-pnictogen anions
The structure, stability, bonding and π-aromaticity in novel cyclic all-pnictogen heterocyclic anions, P2N3− and P3N2−, and in their heavier analogues are studied using quantum mechanical computations.

Phys. Chem. Chem. Phys., 2016,18, 11738-11745
https://doi.org/10.1039/C5CP07236K
Biicosahedral metallaboranes: aromaticity in metal derivatives of three-dimensional analogues of naphthalene
The CpM units in the biicosahedral CpNiB20H17 and CpCoCB19H17 structures energetically prefer to be located at a meta vertex of the biicosahedron. Similar biicosahedral CpFeB20H17 structures are found with an agostic hydrogen atom bridging an Fe–B edge.
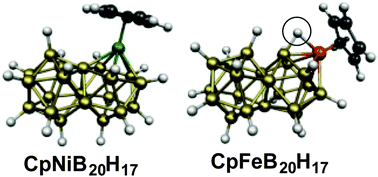
Phys. Chem. Chem. Phys., 2016,18, 11707-11710
https://doi.org/10.1039/C5CP05708F
Centrohexaindane: six benzene rings mutually fixed in three dimensions – solid-state structure and six-fold nitration
The solid-state structure of centrohexaindane, 1, and the sixfold nitration of this three-dimensional polycyclic and topologically nonplanar hydrocarbon is reported.
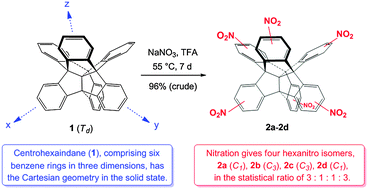
Phys. Chem. Chem. Phys., 2016,18, 11722-11737
https://doi.org/10.1039/C5CP07005H
Unification of ground-state aromaticity criteria – structure, electron delocalization, and energy – in light of the quantum chemical topology
Energy, bond length and electron delocalization are connected within the context of quantum chemical topology theories.
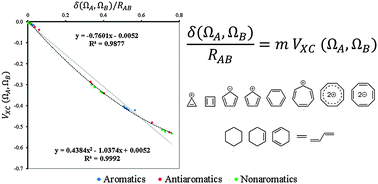
Phys. Chem. Chem. Phys., 2016,18, 11693-11699
https://doi.org/10.1039/C5CP05222J
Towards physical interpretation of substituent effects: the case of meta- and para-substituted anilines
Quantum chemical modeling was used to investigate the electron-donating properties of the amino group in a series of meta- and para-X-substituted anilines (X = NMe2, NH2, OH, OMe, CH3, H, F, Cl, CF3, CN, CHO, COMe, CONH2, COOH, NO2, and NO).
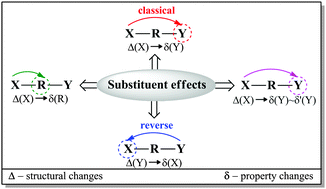
Phys. Chem. Chem. Phys., 2016,18, 11711-11721
https://doi.org/10.1039/C5CP06702B
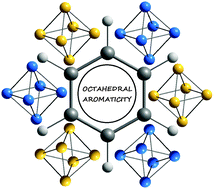
Octahedral aromaticity in 2S+1A1g X6q clusters (X = Li–C and Be–Si, S = 0–3, and q = −2 to +4)
Octahedral aromaticity was found in most clusters of formula X6q (X = Li–C and Be–Si) with q = −2 to +4 and spin states ranging from the singlet to the septet that have electronic configurations of closed-shells or open shells half-filled with the same spin electrons.
Phys. Chem. Chem. Phys., 2016,18, 11700-11706
https://doi.org/10.1039/C5CP07011B
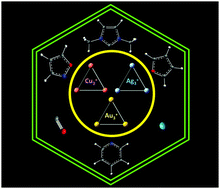
σ-Aromatic cyclic M3+ (M = Cu, Ag, Au) clusters and their complexation with dimethyl imidazol-2-ylidene, pyridine, isoxazole, furan, noble gases and carbon monoxide
The structure, stability, bonding and σ-aromaticity in dimethyl imidazol-2-ylidene, pyridine, isoxazole, furan, noble gas and carbon monoxide bound M3+ (M = Cu, Ag, Au) complexes are analyzed.
Phys. Chem. Chem. Phys., 2016,18, 11661-11676
https://doi.org/10.1039/C5CP06282A
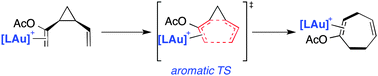
The effect of the metal fragment on the aromaticity and synchronicity of the gold(I)-catalysed divinylcyclopropane–cycloheptadiene rearrangement
The gold(I)-catalysed divinylcyclopropane–cycloheptadiene rearrangement has been studied computationally within the Density Functional Theory framework.
Phys. Chem. Chem. Phys., 2016,18, 11677-11682
https://doi.org/10.1039/C5CP06523B
From linear to cyclic oligoparaphenylenes: electronic and molecular changes traced in the vibrational Raman spectra and reformulation of the bond length alternation pattern
Linear versus cyclic π-electron conjugation is discussed in oligoparaphenylenes.

Phys. Chem. Chem. Phys., 2016,18, 11683-11692
https://doi.org/10.1039/C5CP05500H
Is C50 a superaromat? Evidence from electronic structure and ring current calculations
Of the three lowest isomers of C50 fullerene, the minimal energy D3 isomer comes closest to a spherical aromat or superaromat.

Phys. Chem. Chem. Phys., 2016,18, 11653-11660
https://doi.org/10.1039/C5CP04970A
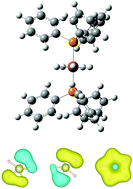
σ-Aromaticity in polyhydride complexes of Ru, Ir, Os, and Pt
Transition-metal hydrides are essential for catalysis, organic synthesis, and hydrogen storage. In this work we study IrH5(PPh3)2, (RuH5(PiPr3)2)−, (OsH5(PiPr3)2)−, and OsH4(PPhMe2)3 polyhydride complexes, where the metal is five-fold coordinated in-plane. The unusual coordination of these compounds can be explained by σ-aromaticity.
Phys. Chem. Chem. Phys., 2016,18, 11644-11652
https://doi.org/10.1039/C5CP04330A
About this collection
Guest Edited by Gabriel Merino and Miquel Solà this collection of articles celebrates the 150th anniversary of the seminal paper ‘‘Sur la constitution des substances aromatiques’’ by August Kekulé and presents a snapshot of present-day research in aromaticity.