Cyclic π-electron delocalization in non-planar linear acenes†
Abstract
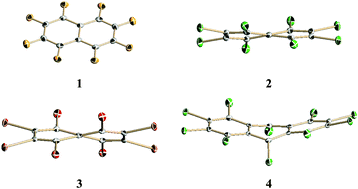
Based on the experimental data of newly determined structures of per-substituted naphthalenes by halogen atoms (F, Cl and Br) and perchloroanthracene, as well as on the molecular modeling we have shown that deviation from planarity leads to relatively small changes in the cyclic π-electron delocalization of acenes. Per-substituted naphthalenes are twisted, whereas perchloroanthracene adopts a boat conformation in the solid state. For the most distorted case – perbromonaphthalene twisted by 34.7°, the geometry-based HOMA index drops down by 0.128 of the unit only, which means ca. 15% reduction of the extent of π-electron delocalization as referred to naphthalene. To account for the changes in the aromatic stabilization energies (ASEs), exaltation of magnetic susceptibilities (Λ) and strain energies (SEs) upon bending we have proposed a set of homodesmotic reactions based on the peri-substituted systems, where the reference compounds have similar conformation to the distorted aromatics. The decrease of aromaticity is smooth, but regular in a rather large range of distortion. Notably, the most extreme case – 1,4,5,8-tetra-t-butylnaphthalene, twisted by 51.7° with an estimated strain energy of 26 kcal mol−1 – is still aromatic. The ASE, Λ and HOMA decrease by 19.1 kcal mol−1, 7 cgs-ppm and 0.34 of the unit, respectively, as referred to naphthalene.
- This article is part of the themed collection: Electron delocalization and aromaticity: 150 years of the Kekulé benzene structure

 Please wait while we load your content...
Please wait while we load your content...