Biicosahedral metallaboranes: aromaticity in metal derivatives of three-dimensional analogues of naphthalene†
Abstract
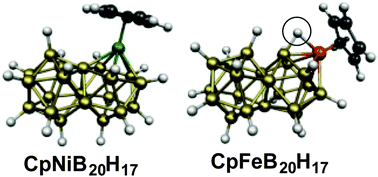
Consideration of the well-known very stable icosahedral B12H122− as a three-dimensional analogue of benzene was extended by the recent synthesis of the biicosahedral B21H18− as a three-dimensional analogue of naphthalene. The preferred structures of metallaboranes derived from B21H18− have now been examined by density functional theory. The isoelectronic species CpNiB20H17 and CpCoCB19H17 have the 46 skeletal electrons expected by the Wade-Mingos and Jemmis rules for a structure consisting of two face-sharing fused icosahedra. The CpM units in these structures energetically prefer to be located at a meta vertex of the biicosahedron. The analogous ferraboranes CpFeB20H17 with only 44 skeletal electrons also have related biicosahedral structures. The presence of an agostic hydrogen atom bridging an Fe–B edge compensates for the two-electron deficiency in CpFeB20H17 relative to CpNiB20H17. The nucleus-independent chemical shift (NICS) values of these systems indicate them to be strongly aromatic.
- This article is part of the themed collection: Electron delocalization and aromaticity: 150 years of the Kekulé benzene structure

 Please wait while we load your content...
Please wait while we load your content...