Aromatic character of planar boron-based clusters revisited by ring current calculations†
Abstract
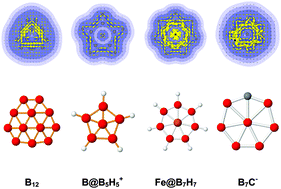
The planarity of small boron-based clusters is the result of an interplay between geometry, electron delocalization, covalent bonding and stability. These compounds contain two different bonding patterns involving both σ and π delocalized bonds, and up to now, their aromaticity has been assigned mainly using the classical (4N + 2) electron count for both types of electrons. In the present study, we reexplored the aromatic feature of different types of planar boron-based clusters making use of the ring current approach. B3+/−, B42−, B5+/−, B6, B7−, B82−, B9−, B102−, B11−, B12, B13+, B142− and B162− are characterized by magnetic responses to be doubly σ and π aromatic species in which the π aromaticity can be predicted using the (4N + 2) electron count. The triply aromatic character of B12 and B13+ is confirmed. The π electrons of B182−, B19− and B202− obey the disk aromaticity rule with an electronic configuration of [1σ21π41δ42σ2] rather than the (4N + 2) count. The double aromaticity feature is observed for boron hydride cycles including B@B5H5+, Li7B5H5 and M@BnHnq clusters from both the (4N + 2) rule and ring current maps. The double π and σ aromaticity in carbon-boron planar cycles B7C−, B8C, B6C2, B9C−, B8C2 and B7C3− is in conflict with the Hückel electron count. This is also the case for the ions B11C5+/− whose ring current indicators suggest that they belong to the class of double aromaticity, in which the π electrons obey the disk aromaticity characteristics. In many clusters, the classical electron count cannot be applied, and the magnetic responses of the electron density expressed in terms of the ring current provide us with a more consistent criterion for determining their aromatic character.
- This article is part of the themed collection: Electron delocalization and aromaticity: 150 years of the Kekulé benzene structure

 Please wait while we load your content...
Please wait while we load your content...