Centrohexaindane: six benzene rings mutually fixed in three dimensions – solid-state structure and six-fold nitration†
Abstract
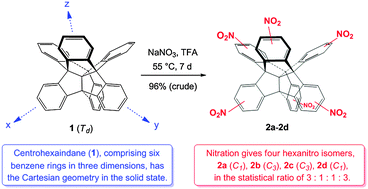
The solid-state molecular structure of centrohexaindane (1), a unique hydrocarbon comprising six benzene rings clamped to each other in three dimensions around a neopentane core, and the molecular packing in crystals of 1·CHCl3 are reported. The molecular Td-symmetry and the Cartesian orientation of the six indane wings of 1 in the solid state have been confirmed. The course and limitation of electrophilic aromatic substitution of 1 are demonstrated for the case of nitration. Based on nitration experiments of a lower congener of 1, tribenzotriquinacene 5, the six-fold nitrofunctionalisation of 1 has been achieved in excellent yield, giving four constitutional isomers, two nonsymmetrical (14 and 17) and two C3-symmetrical ones (15 and 16), all of which contain one single nitro group in each of the six benzene rings. The relative yields of the four isomers (∼3 : 1 : 1 : 3) point to a random electrophilic attack of the electrophiles at the twelve formally equivalent outer positions of the aromatic periphery of 1, suggesting electronic independence of its six aromatic π-electron systems. In turn, the pronounced conformational rigidity of the centrohexacyclic framework of 1 enables the unequivocal structural identification of the isomeric hexanitrocentrohexaindanes 14–17 by 1H NMR spectroscopy.
- This article is part of the themed collection: Electron delocalization and aromaticity: 150 years of the Kekulé benzene structure

 Please wait while we load your content...
Please wait while we load your content...