Themed collection Editor’s choice – Andrei Yudin

Nickel-catalyzed C–N bond activation: activated primary amines as alkylating reagents in reductive cross-coupling
The reductive cross coupling of pyridinium salts derived from readily available primary alkyl amines with aryl halides has been achieved under mild reaction conditions using a nickel catalyst.
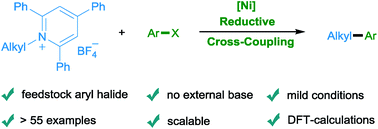
Chem. Sci., 2019,10, 4430-4435
https://doi.org/10.1039/C9SC00783K
Chiral phosphoric acid-catalyzed enantioselective construction of structurally diverse benzothiazolopyrimidines
Chiral phosphoric acid catalyzed the formal [4+2]-cycloaddition of 2-benzothiazolimines with enecarbamates to provide benzothiazolopyrimidines with up to 99% yield and >99% ee.
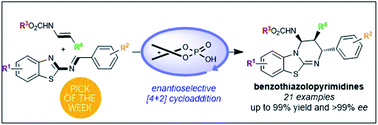
Chem. Sci., 2019,10, 3765-3769
https://doi.org/10.1039/C8SC05581E
Dinuclear manganese alkoxide complexes as catalysts for C–N bond cleavage of simple tertiary N,N-dialkylamides to give esters
Amide bonds are stable due to the resonance between the nitrogen lone pair and the carbonyl moiety, and therefore the chemical transformation of amides, especially tertiary amides, involving C–N bond fission is considered one of the most difficult organic reactions, unavoidably requiring harsh reaction conditions and strong acids or bases.
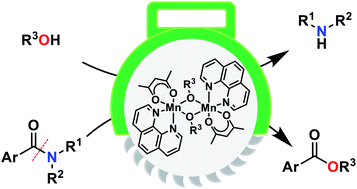
Chem. Sci., 2019,10, 2860-2868
https://doi.org/10.1039/C8SC05819A
Catalytic β C–H amination via an imidate radical relay
An iodine-catalyzed strategy for β C–H amination of alcohols is enabled by a chemo-, regio-, and stereo-selective H-atom transfer mechanism.
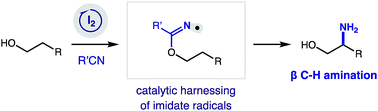
Chem. Sci., 2019,10, 2693-2699
https://doi.org/10.1039/C8SC05685D
Macrocyclisation of small peptides enabled by oxetane incorporation
Head-to-tail peptide macrocyclisations are significantly improved, as measured by isolated yields, reaction rates and product distribution, by substitution of one of the backbone amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds with an oxetane ring.
O bonds with an oxetane ring.
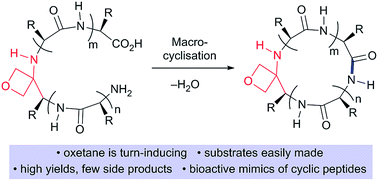
Chem. Sci., 2019,10, 2465-2472
https://doi.org/10.1039/C8SC05474F
Asymmetric synthesis of (−)-naltrexone
The asymmetric synthesis of (−)-naltrexone was achieved by a Rh(I)-catalyzed C–H alkenylation and torquoselective electrocyclization cascade and late-stage C–H hydroxylation.
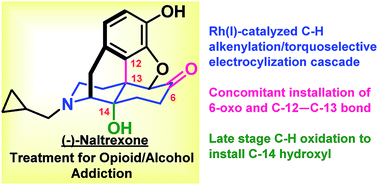
Chem. Sci., 2019,10, 535-541
https://doi.org/10.1039/C8SC03748E
Using stereoretention for the synthesis of E-macrocycles with ruthenium-based olefin metathesis catalysts
The synthesis of E-macrocycles is achieved using stereoretentive, Ru-based olefin metathesis catalysts supported by dithiolate ligands.
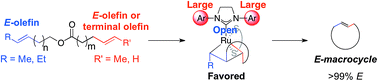
Chem. Sci., 2018,9, 3580-3583
https://doi.org/10.1039/C8SC00435H
Synthetic fermentation of β-peptide macrocycles by thiadiazole-forming ring-closing reactions
A new thiadiazole-forming macrocyclization reaction enables the one-pot synthesis of cyclic β-peptide libraries from readily accessible building blocks without additional reagents.

Chem. Sci., 2018,9, 2159-2167
https://doi.org/10.1039/C7SC05057G
Mechanistic insights into boron-catalysed direct amidation reactions
The generally accepted monoacyloxyboron mechanism of boron-catalysed direct amidation is brought into question in this study, and new alternatives are proposed.
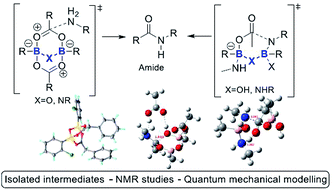
Chem. Sci., 2018,9, 1058-1072
https://doi.org/10.1039/C7SC03595K
Formal aromaticity transfer for palladium-catalyzed coupling between phenols and pyrrolidines/indolines
A formal aromaticity transfer reaction between phenols and pyrrolines/indolines has been developed: a redox- and atom-efficient method to synthesize N-cyclohexylpyrroles/indoles.
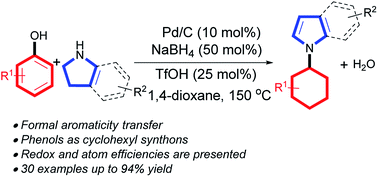
Chem. Sci., 2017,8, 6954-6958
https://doi.org/10.1039/C7SC02578E
About this collection
In 2019, Chemical Science welcomed Professor Andrei Yudin to the journal as an Associate Editor, handling papers in the areas of organic synthesis, transition metal catalysis, peptide preparation and macrocyclisation.
Andrei has looked back over recently published Chemical Science papers and has selected some outstanding articles on the theme of catalysis and synthetic methodology. We hope you enjoy reading through this selection.