Nickel-catalyzed C–N bond activation: activated primary amines as alkylating reagents in reductive cross-coupling†
Abstract
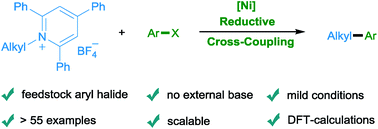
Nickel-catalyzed reductive cross coupling of activated primary amines with aryl halides under mild reaction conditions has been achieved for the first time. Due to the avoidance of stoichiometric organometallic reagents and external bases, the scope regarding both coupling partners is broad. Thus, a wide range of substrates, natural products and drugs with diverse functional groups are tolerated. Moreover, experimental mechanistic investigations and density functional theory (DFT) calculations in combination with wavefunction analysis have been performed to understand the catalytic cycle in more detail.
- This article is part of the themed collections: Most popular 2019-2020 catalysis articles, Most popular 2018-2019 catalysis articles, 2019 International Open Access Week Collection and Editor’s choice – Andrei Yudin


 Please wait while we load your content...
Please wait while we load your content...