Chiral phosphoric acid-catalyzed enantioselective construction of structurally diverse benzothiazolopyrimidines†
Abstract
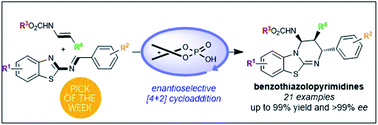
A highly efficient catalytic enantioselective [4+2] cycloaddition was developed between 2-benzothiazolimines and enecarbamates. A wide range of benzothiazolopyrimidines bearing three contiguous stereogenic centers was obtained in high to excellent yields and with excellent diastereo- and enantioselectivities (d.r. > 98 : 2 and up to >99% ee). Furthermore, this chiral phosphoric acid-catalyzed strategy was scalable and enabled access to a new class of optically pure Lewis base isothiourea derivatives.
- This article is part of the themed collections: Most popular 2019-2020 catalysis articles, New reactivity in organic chemistry, 2019 Chemical Science HOT Article Collection, Editor’s choice – Andrei Yudin and 2019 ChemSci Pick of the Week Collection


 Please wait while we load your content...
Please wait while we load your content...