Themed collection Recent advances in redox-flow batteries

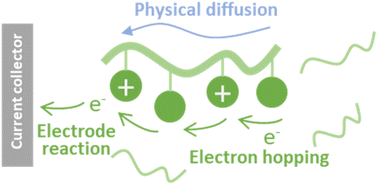
Charge-transport kinetics of dissolved redox-active polymers for rational design of flow batteries
Electrochemical kinetics of dissolved redox-active polymers are formulated. The model can be used for the rational design of redox-flow cells.
RSC Adv., 2023,13, 547-557
https://doi.org/10.1039/D2RA07208D
An ultra-stable reference electrode for scaled all-vanadium redox flow batteries
An ultra-stable reference electrode with novel design has been developed for scaled all-vanadium redox flow batteries, which demonstrates high accuracy and long-term stability over 500 charge–discharge cycles.

RSC Adv., 2022,12, 32173-32184
https://doi.org/10.1039/D2RA05781F
Structure–activity correlation of thermally activated graphite electrodes for vanadium flow batteries
Structural changes on the surface of graphite felts after thermal activation were monitored. Fundamental correlations led to a new model to explain the morphological evolution and its effects on the electrocatalytic activity in vanadium flow batteries.

RSC Adv., 2022,12, 14119-14126
https://doi.org/10.1039/D2RA02368G
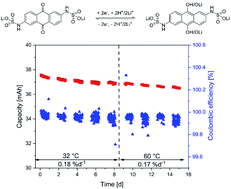
Anthraquinone-2,6-disulfamidic acid: an anolyte with low decomposition rates at elevated temperatures
A new anthraquinone based anolyte material for redox flow batteries revealed an extraordinarily high stability at elevated electrolyte temperatures.
RSC Adv., 2021,11, 38759-38764
https://doi.org/10.1039/D1RA05545C
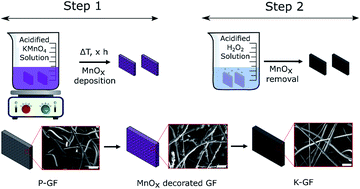
Rapid wet-chemical oxidative activation of graphite felt electrodes for vanadium redox flow batteries
Schematic diagram of the K-GF fabrication process. Step 1: deposition of MnOx layers onto the P-GF electrode surface using acidified KMnO4 solutions. Step 2: removal of MnOx layers using an acidified H2O2 solution to produce the K-GF electrode.
RSC Adv., 2021,11, 32095-32105
https://doi.org/10.1039/D1RA05808H
Performance and stability comparison of Aemion™ and Aemion+™ membranes for vanadium redox flow batteries
Cycling behaviour of Aemion™ (50 μm), Aemion+™ (50 μm), Aemion+™ (15 μm) and Nafion® 212 (50 μm) at 100 mA cm−2. (a) Coulombic efficiency, (b) energy efficiency and (c) membrane resistance.

RSC Adv., 2021,11, 13077-13084
https://doi.org/10.1039/D1RA01079D
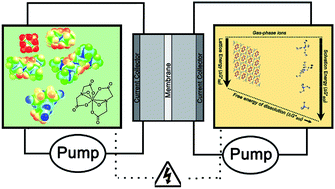
Designing high energy density flow batteries by tuning active-material thermodynamics
With the cost of renewable energy near parity with fossil fuels, energy storage is paramount. We report a breakthrough on a bioinspired NRFB active-material, with greatly improved solubility, and place it in a predictive theoretical framework.
RSC Adv., 2021,11, 5432-5443
https://doi.org/10.1039/D0RA10913D
High energy density electrolytes for H2/Br2 redox flow batteries, their polybromide composition and influence on battery cycling limits
Polybromides formation in aqueous bromine electrolytes and influence on H2/Br2 redox flow battery performance is investigated the first time.
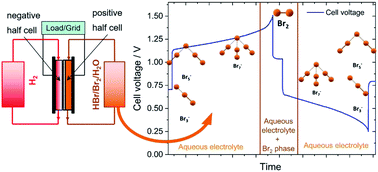
RSC Adv., 2021,11, 5218-5229
https://doi.org/10.1039/D0RA10721B
Alkaline all iron redox flow battery with a polyethylene/poly(styrene-co-divinylbenzene) interpolymer cation-exchange membrane
A polyethylene styrene–DVB interpolymer cation exchange membrane is reported for use in a highly alkaline all iron redox flow battery.
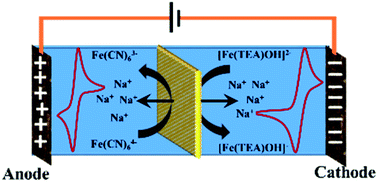
RSC Adv., 2020,10, 44824-44833
https://doi.org/10.1039/D0RA08316J
Porous carbon–carbon composite electrodes for vanadium redox flow batteries synthesized by twin polymerization
Synthesis, characterization and electrochemical evaluation of composite electrodes – synthesized via twin polymerization – for utilization in vanadium redox flow batteries.

RSC Adv., 2020,10, 41926-41935
https://doi.org/10.1039/D0RA07741K
Enhancing the solubility of 1,4-diaminoanthraquinones in electrolytes for organic redox flow batteries through molecular modification
The redox-active 1,4-diaminoanthraquinone structure was modified with several side chains in order to increase the solubility in organic electrolytes for redox flow batteries.
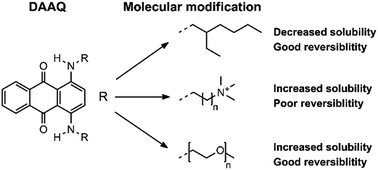
RSC Adv., 2020,10, 39601-39610
https://doi.org/10.1039/D0RA06851A
Resolving charge-transfer and mass-transfer processes of VO2+/VO2+ redox species across the electrode/electrolyte interface using electrochemical impedance spectroscopy for vanadium redox flow battery
Effect of redox species concentration across the electrode/electrolyte interface on the EIS features.

RSC Adv., 2020,10, 30887-30895
https://doi.org/10.1039/D0RA05224H
Fundamental properties of TEMPO-based catholytes for aqueous redox flow batteries: effects of substituent groups and electrolytes on electrochemical properties, solubilities and battery performance
The effects of substituent groups of TEMPO-based catholytes and supporting electrolytes on electrochemical properties, solubility and battery performance were examined systematically for aqueous redox flow batteries.

RSC Adv., 2020,10, 21839-21844
https://doi.org/10.1039/D0RA03424J
Preparation of a porous graphite felt electrode for advance vanadium redox flow batteries
A graphite felt electrode with unique porous structure was designed to improve the performance of VRFBs.

RSC Adv., 2020,10, 13374-13378
https://doi.org/10.1039/D0RA00666A
Fabrication of a composite anion exchange membrane with aligned ion channels for a high-performance non-aqueous vanadium redox flow battery
Fabrication of high-conductivity ion exchange membranes (IEMs) is crucial to improve the performance of non-aqueous vanadium redox flow batteries (NAVRFBs).

RSC Adv., 2020,10, 5010-5025
https://doi.org/10.1039/C9RA08616A
Solubility model of metal complex in ionic liquids from first principle calculations
A predictive model based on first principles calculations has been proposed to study the solid–liquid equilibria comprising of metal complexes and ionic liquids.
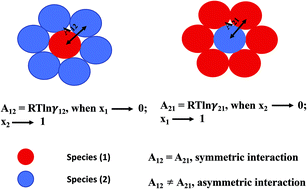
RSC Adv., 2019,9, 18506-18526
https://doi.org/10.1039/C9RA04042K
An all organic redox flow battery with high cell voltage
An all organic redox flow battery shows a high OCV of 2.97 V and average coulombic efficiency 72% over 95 cycles.

RSC Adv., 2019,9, 13128-13132
https://doi.org/10.1039/C9RA01514K
Aqueous dispersions of carbon black and its hybrid with carbon nanofibers
Optimal hybrid dispersion of carbon black (CB) and nanofibers (CNFs) is formed at a critical content of CNFs before its aggregation concentration so that CNFs wire CB aggregates to recover the conductivity loss without increasing of CB rigidity.

RSC Adv., 2018,8, 32119-32131
https://doi.org/10.1039/C8RA05446K
Polybenzimidazole/Nafion hybrid membrane with improved chemical stability for vanadium redox flow battery application
Novel polybenzimidazole (PBI)/Nafion hybrid membranes for the VRFB are made by spray coating a Nafion layer to protect PBI from chemical degradation.

RSC Adv., 2018,8, 25304-25312
https://doi.org/10.1039/C8RA03921F
Pyridyl group design in viologens for anolyte materials in organic redox flow batteries
Organic redox compounds represent an emerging class of active materials for organic redox-flow batteries (RFBs), which are highly desirable for sustainable electrical energy storage.
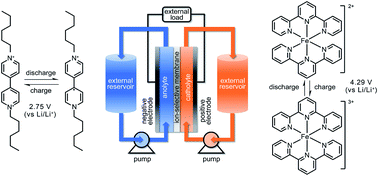
RSC Adv., 2018,8, 18762-18770
https://doi.org/10.1039/C8RA02641F
The effect of adding Bi3+ on the performance of a newly developed iron–copper redox flow battery
In this paper, we propose a new, abundant, cost-effective, non-toxic, and environmentally benign iron–copper redox flow battery (Fe/Cu RFB), which employs Fe2+/Fe3+ and Cu+/Cu0 as the positive and negative electrolytes, respectively.

RSC Adv., 2018,8, 8537-8543
https://doi.org/10.1039/C7RA12926B
Unbiased, complete solar charging of a neutral flow battery by a single Si photocathode
Solar redox flow batteries have attracted attention as a possible integrated technology for simultaneous conversion and storage of solar energy.
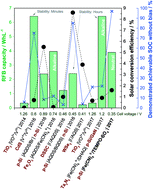
RSC Adv., 2018,8, 6331-6340
https://doi.org/10.1039/C8RA00319J
About this collection
Redox flow batteries play a crucial role in the decarbonization of the energetic economy. As energy sources move to greener alternatives, such as solar or wind power, the need to store electric energy becomes imperative given the intermittent nature of these sources. Redox flow batteries are highly promising for use in large scale grid energy storage due to various factors. These include the ability to size them independently for power and energy needs, their high efficiency, operation at room temperature, and an exceptionally long charge and discharge cycle life.
Currently, the most extensively studied and commercially utilized variants are all-vanadium redox batteries. Nevertheless, numerous research initiatives that focus on exploring other redox couples are actively progressing, which include different metal couples, organic molecules both in aqueous and non-aqueous media, or hybrid systems combining soluble redox chemistry with other types of reactions.
In this themed collection, we showcase relevant and interesting research papers published in RSC Advances during the last 5 years. The purpose of this collection is to show recent advances in our understanding of the different aspects that affect redox-flow battery performance, as well as new strategies and proofs of concept to advance the technology. Among these articles, electrode materials and architecture, membrane chemistry, electrolyte formulation and different organic and inorganic redox couples are explored. This collection encompasses fundamental, synthetic and application studies.