Themed collection Main Group Transformations

Main group transformations
Welcome to this themed issue of Dalton Transactions focusing on main group transformations.

Dalton Trans., 2016,45, 5879-5879
https://doi.org/10.1039/C6DT90027E
Cyclodiphosphazanes: options are endless
This short review describes the transition metal chemistry of cyclodiphosphazanes.
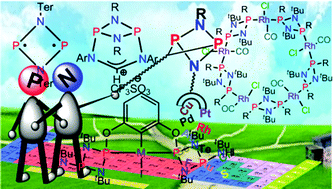
Dalton Trans., 2016,45, 12252-12282
https://doi.org/10.1039/C6DT01121G
Inorganic sulfur–nitrogen compounds: from gunpowder chemistry to the forefront of biological signaling
History, chemical properties and emerging biology of HSNO/SNO−, SSNO− and [ONN(O)-SO3]2− and their role in the sulfide/nitric oxide cross talk.
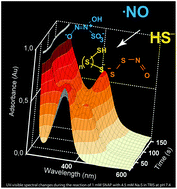
Dalton Trans., 2016,45, 5908-5919
https://doi.org/10.1039/C5DT05034K
A very peculiar family of N-heterocyclic phosphines: unusual structures and the unique reactivity of 1,3,2-diazaphospholenes
The N-heterocyclic phosphines portrayed gain unusual chemical reactivity from the ionic polarization of exocyclic P–X bonds or low-energy P–P bond cleavage.

Dalton Trans., 2016,45, 5896-5907
https://doi.org/10.1039/C6DT00085A
25 years of N-heterocyclic carbenes: activation of both main-group element–element bonds and NHCs themselves
Expanding the belt! NHC ring expansion reactions and E–E activation from the 1960s till the present are summarized.
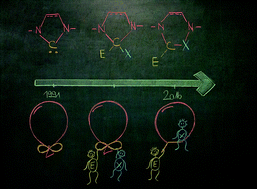
Dalton Trans., 2016,45, 5880-5895
https://doi.org/10.1039/C5DT04106F
B(C6F5)3-catalyzed metal-free hydrogenation of 3,6-diarylpyridazines
Metal-free hydrogenation of 3,6-diarylpyridaxines was realized for the first time to furnish 1,4,5,6-tetrahydropyridazine derivatives in 85–95% yields.
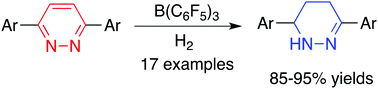
Dalton Trans., 2016,45, 5945-5948
https://doi.org/10.1039/C5DT03872C
Abnormal carbene–silicon halide complexes
Reaction of the anionic NHDC ligand, [:C{[N(2,6-Pri2C6H3)]2CHCLi}]n (1), with SiCl4 gives the trichlorosilyl-substituted NHC ligand (7). Abnormal carbene–SiCl4 complex (8) can be conveniently synthesized by combining 7 with HCl·NEt3. Meanwhile, 7 may react with CH2Cl2 in warm hexane, giving the abnormal carbene-complexed SiCl3+ cation (9). The structure and bonding of 9 have also been probed by DFT computations.
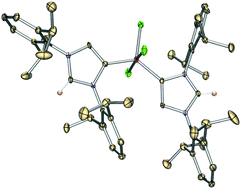
Dalton Trans., 2016,45, 5941-5944
https://doi.org/10.1039/C5DT03894D
N-Heterocyclic olefin stabilized boron dication
Boron mono- and di-cations featuring a nucleophilic N-heterocyclic olefin and the pentamethylcyclopentadienyl substituent have been prepared and structurally characterized. Experimental and theoretical investigations show that [η5-Cp*B-NHO]2+ is considerably more Lewis acidic than [η5-Cp*B-IMes]2+ due to the steric congestion imposed by the bent geometry of NHO around the central boron atom.
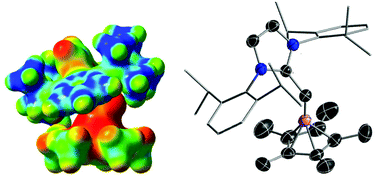
Dalton Trans., 2016,45, 5937-5940
https://doi.org/10.1039/C5DT03847B
Reaction of an allylstannylene with adamantyl phosphaalkyne
The reaction of a terphenyl tin(II) compound bearing an η3 coordinating allyl ligand with adamantyl phosphaalkyne is reported.
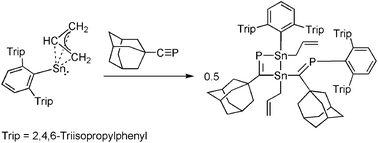
Dalton Trans., 2016,45, 5933-5936
https://doi.org/10.1039/C5DT03792A
Lewis acid–base 1,2-addition reactions: synthesis of pyrylium borates from en-ynoate precursors
Treatment of methyl (Z)-2-alken-4-ynoates with the strong Lewis acid tris(pentafluorophenyl) borane, B(C6F5)3, yield 2,5,6-substituted zwitterionic pyrylium borate species via an intramolecular 6-endo-dig cyclisation reaction.

Dalton Trans., 2016,45, 5929-5932
https://doi.org/10.1039/C5DT03340C
Facile kinetic induction of a dihydropyridide to pyrrolide ring contraction
A sterically demanding N-aryl carbodiimide reacts with magnesium 1,4-dihydropyridides to initiate heterocyclic ring contraction and pyrrolide formation under unprecedentedly mild conditions.
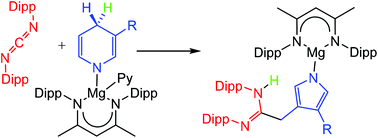
Dalton Trans., 2016,45, 5925-5928
https://doi.org/10.1039/C5DT02887F
Antiproliferative activity of (η6-arene)ruthenacarborane sandwich complexes against HCT116 and MCF7 cell lines
The [(η6-arene)RuC2B9H11] complexes (arene = p-cymene (2), biphenyl (3) and 1-Me-4-COOEt-C6H4 (4)) show cytotoxic activity and excellent selectivity towards specific tumour cells.
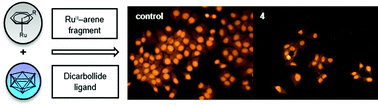
Dalton Trans., 2017,46, 12067-12080
https://doi.org/10.1039/C7DT02027A
N-Heterocyclic carbene adducts of the heavier group 15 tribromides. Normal to abnormal isomerism and bromide ion abstraction
The reactivity of the heavier group 15 tribromides, SbBr3 and BiBr3, towards 1,3-bis(2,6-diisopropylphenyl)-imidazol-2-ylidene (IPr) is described.
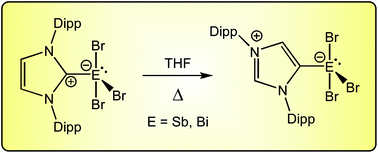
Dalton Trans., 2017,46, 12053-12066
https://doi.org/10.1039/C7DT02431B
Homopolar dihydrogen bonding in ligand stabilized diberyllium hydride complexes, Be2(CH3)2H2L2 (L = H−, CO, N-heterocyclic carbene and CN−)
Diberyllium hydride complex – not a diborane analogue: the diberyllium hydrides Be2(CH3)2H2L2 (L = H−, CO, NHC and CN−) are isostructural to diborane but differ in the nature of the bonding interaction at the bridging H-atoms.
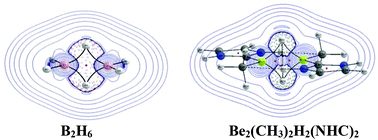
Dalton Trans., 2016,45, 7836-7846
https://doi.org/10.1039/C5DT04293C
Variable coordination modes and catalytic dehydrogenation of B-phenyl amine–boranes
The binding mode of B-aryl substituted amine–boranes at {Rh(bisphoshine)}+ fragments can manipulated by variation of the P–Rh–P bite-angle.
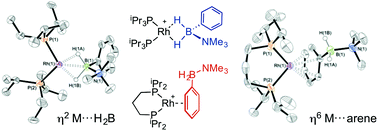
Dalton Trans., 2016,45, 6183-6195
https://doi.org/10.1039/C6DT00197A
Synthesis of mono-, di-, and triaminobismuthanes and observation of C–C coupling of aromatic systems with bismuth(III) chloride
Mono-, di- or triaminobismuthanes were synthesized depending on the sterical demand of the organic substituent. In one case, a C–C coupling product was observed as main product in the reaction of BiCl3 with Mes*(SiMe3)NLi.
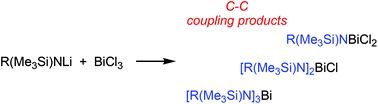
Dalton Trans., 2016,45, 6053-6059
https://doi.org/10.1039/C6DT00229C
Tin-catalyzed hydrophosphination of alkenes
Simple tin derivatives, Cp*2SnCl2 (1) and Ph2SnCl2 (2), catalyze the hydrophosphination of alkene substrates with diphenylphosphine.
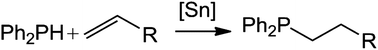
Dalton Trans., 2016,45, 6204-6209
https://doi.org/10.1039/C5DT04272K
Synthesis and reactivity of fluorenyl-tethered N-heterocyclic stannylenes
N-Heterocyclic stannylenes containing a functionalised donor arm have been synthesised using a transamination strategy from [Sn{N(SiMe3)2}2] and fluorenyl-tethered diamines.
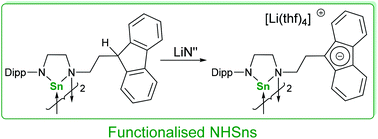
Dalton Trans., 2016,45, 6282-6293
https://doi.org/10.1039/C5DT04060D
Cyclic NHC-stabilized silylphosphinoalanes and -gallanes
The reaction between prestabilized group 13 metal hydrides and primary silylphosphines lead to the formation of rare, butterfly-like folded, four membered rings including free lone pairs on the phosphorus atoms, wherein the Lewis base can readily be substituted by N-heterocyclic carbenes.
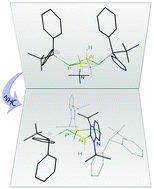
Dalton Trans., 2016,45, 6275-6281
https://doi.org/10.1039/C5DT03889H
Accessible heavier s-block dihydropyridines: structural elucidation and reactivity of isolable molecular hydride sources
Transmetallation of lithiodihydropyridines with Group 1 alkoxides provides facile access to reactive MH (M = Na, K) sources, which show significant structural diversity due in part to the distinct ways that Na/K engage with the σ (green) and π (red) donor systems of the DHP ligands.
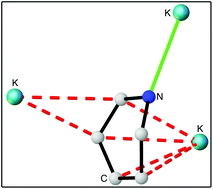
Dalton Trans., 2016,45, 6234-6240
https://doi.org/10.1039/C5DT04224K
Pyridinium–phosphonium dications: highly electrophilic phosphorus-based Lewis acid catalysts
Using commercially available 2-pyridyldiphenylphosphine (o-NC5H4)PPh2, a family of electrophilic phosphonium cations [(o-NC5H4)PFPh2]+ (2) and dications [(o-MeNC5H4)PRPh2]2+ (R = F (4); Me (5)) were prepared.
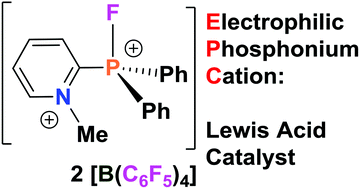
Dalton Trans., 2016,45, 5949-5957
https://doi.org/10.1039/C5DT03796D
Monoorganoantimony(V) phosphonates and phosphoselininates
Synthesis and structural characterization of novel tetra- and dinuclear organoantimony oxo-hydroxo clusters ligated by phosphonates and phosphonates/selininates are reported. Mass spectral studies reveal that the solid state molecular structures of these clusters were retained in solution as well.
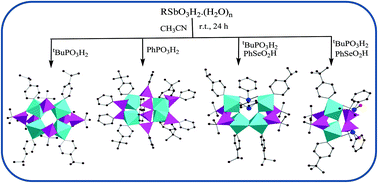
Dalton Trans., 2016,45, 6269-6274
https://doi.org/10.1039/C5DT03449C
Blending materials composed of boron, nitrogen and carbon to transform approaches to liquid hydrogen stores
Mixtures of hydrogen storage materials are examined to find a ‘fuel blend’ that remains a liquid phase throughout hydrogen release, maximizes hydrogen storage density, minimizes impurities and is thermally stable.
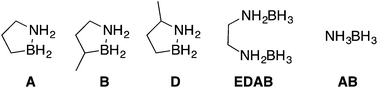
Dalton Trans., 2016,45, 6196-6203
https://doi.org/10.1039/C5DT04276C
Amino group combined P/Ge and P/Sn Lewis pairs: synthesis and dipolar addition reactions to alkyne and aldehyde molecules
Amino group combined P/Ge FLPs Ph2PN(R)GeCl3 (R = 2,6-iPr2C6H3 (1), 2,4,6-Me3C6H2 (2), and C6H11 (3)) and Ph2PN(2,6-iPr2C6H3)GeMe3 (4) as well as P/Sn FLP Ph2PN(2,6-iPr2C6H3)SnMe3 (5) were prepared and utilized for reactions with alkyne and aldehyde molecules.
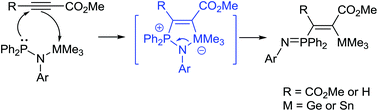
Dalton Trans., 2016,45, 6259-6268
https://doi.org/10.1039/C5DT03873A
Synthetic and reactivity studies of hetero-tri-anionic sodium zincates
The blue, red and green spheres represent three different anions present within a series of novel hetero-trianionic sodium zincates. The syntheses and structures of the complexes are reported as well as their reactivities with important organic molecules.
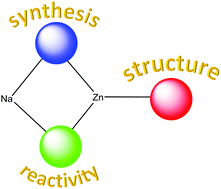
Dalton Trans., 2016,45, 6222-6233
https://doi.org/10.1039/C5DT04329H
A zwitterionic triphosphenium compound as a tunable multifunctional donor
We present a zwitterionic triphosphenium molecule which features dicoordinate, tricoordinate and tetracoordinate phosphorus centers in addition to a cyclopentadienyl moiety. Crystallographic, computational, electrochemical and spectroscopic data illustrate and rationalize the reactivity of this molecule as a multifunctional ligand for both transition metals and main group acceptors and suggest how the donor properties may be tuned.
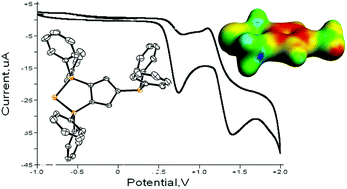
Dalton Trans., 2016,45, 6251-6258
https://doi.org/10.1039/C5DT03915K
The role of imidoselenium(II) chlorides in the formation of cyclic selenium imides via cyclocondensation
Imidoselenium(II) chlorides ClSe[N(tBu)Se]nCl (n = 1–3) are intermediates in the formation of cyclic selenium imides by the reaction of SeCl2 and tBuNH2 in THF.
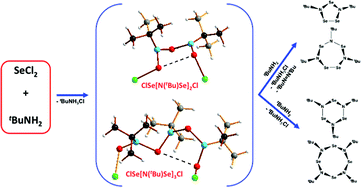
Dalton Trans., 2016,45, 6210-6221
https://doi.org/10.1039/C5DT04236D
Assessing the reactivity of sodium alkyl-magnesiates towards quinoxaline: single electron transfer (SET) vs. nucleophilic alkylation processes
Structurally tracking the reaction of a sodium butylmagnesiate supported by a bulky silyl(bisamide) ligand towards quinoxaline, SET reactivity.
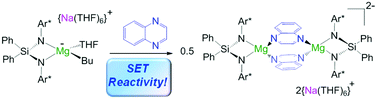
Dalton Trans., 2016,45, 6175-6182
https://doi.org/10.1039/C5DT04044B
Hydroamination of diphenylbutadiyne with secondary N-methyl-anilines using the dipotassium tetrakis(2,6-diisopropylanilino)calciate precatalyst
The s-block metal complex [K2Ca{N(H)Dipp}4] represents a suitable catalyst for the regioselective catalytic single and two-fold hydroamination of diphenylbutadiyne with N-methyl-anilines.
Dalton Trans., 2016,45, 6241-6250
https://doi.org/10.1039/C5DT03818A
Functionalized alkynyl-chlorogermanes: hydrometallation, Ge–Cl bond activation, Ge–H bond formation and chlorine-tert-butyl exchange via a transient germyl cation
An Al–Cl functionalized germane with an activated Ge–Cl bond showed a tBu/Cl exchange via a transient germyl cation.
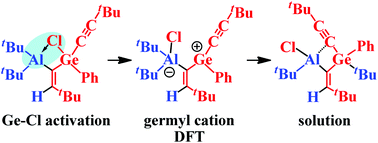
Dalton Trans., 2016,45, 6159-6174
https://doi.org/10.1039/C5DT03918E
Group 13 metal complexes containing the bis-(4-methylbenzoxazol-2-yl)-methanide ligand
On the basis of the deprotonated bis-(4-methylbenzoxazol-2-yl)-methane ligand 1 a series of group 13 metal complexes was synthesised and fully characterised. A detailed comparison of solid state structures demonstrates clearly the similarity of methanide and the omnipresent nacnac ligand.
Dalton Trans., 2016,45, 6149-6158
https://doi.org/10.1039/C5DT03913D
Bis-(benzothiazol-2-yl)-amines and their metal amides: a structural comparison in the solid state
A series of functionalised bis-(benzothiazol-2-yl)-amine ligands and the related dimethylaluminium amides were synthesised and characterised. The solid state structural comparison reveals various coordination motifs, specific folding parameters and hydrogen bonding patterns in detail and guide the way to future Janus head ligand design.

Dalton Trans., 2016,45, 6136-6148
https://doi.org/10.1039/C5DT03911H
Exploring the reducing role of boron: added insights from theory
Why are boron containing systems so effective at CO coupling? Full quantum chemical calculations with density functional theory (DFT) provide interesting insights into why recently reported CO coupling by diboryne systems is such a facile process.
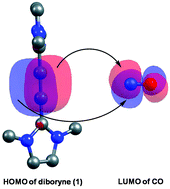
Dalton Trans., 2016,45, 5978-5988
https://doi.org/10.1039/C5DT03799A
Unsolvated Al(C6F5)3: structural features and electronic interaction with ferrocene
The unsolvated Al(C6F5)3 exists as a dimer in the solid state via double Al⋯(ortho-)F bridging and forms a stable adduct with Cp2Fe through η1-coordination.
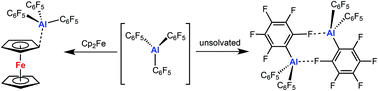
Dalton Trans., 2016,45, 6105-6110
https://doi.org/10.1039/C5DT03895B
Smallest molecular chalcogenidometalate anions of the heaviest metals: syntheses, structures, and their interconversion
Smallest chalcogenidometalate anions of the heaviest metals are presented alongside calculated energetics of these heaviest homologues of [NOx]−, [PO4]2− and [CO3]2−.
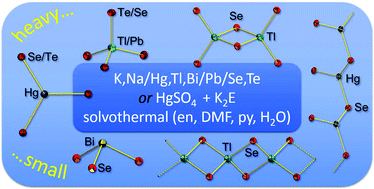
Dalton Trans., 2016,45, 5958-5967
https://doi.org/10.1039/C5DT03996G
Reversible hydrogen activation by a bulky haloborane based FLP system
The FLP species bis(2-(TMP)phenyl)chloroborane was prepared as a monomeric Frustrated Lewis Pair displaying no B–N interaction. Species 1 reacts with H2 to generate reversibly the zwitterionic H2 activation product.
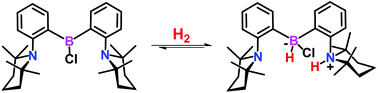
Dalton Trans., 2016,45, 6129-6135
https://doi.org/10.1039/C5DT03916A
Microwave-assisted FLP-catalyzed hydrogenations
Microwave-irradiation accelerates FLP-catalyzed hydrogenations.
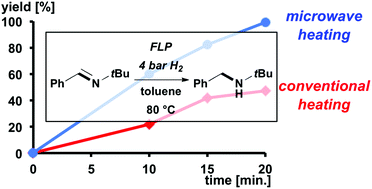
Dalton Trans., 2016,45, 6124-6128
https://doi.org/10.1039/C5DT03857J
Toward highly efficient blue organic light-emitting diodes: fabricating a good-quality emissive layer cast from suitable solvents
Cast from chlorobenzene, a light-emitting layer shows its best morphology and highest efficiency of 18.99 cd A−1 in single-layer FIrpic-based OLEDs.
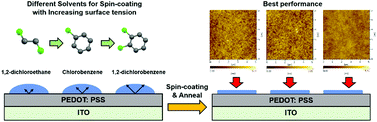
Dalton Trans., 2016,45, 6118-6123
https://doi.org/10.1039/C5DT03776J
Unusual borane addition to conjugated dienylphosphanes under frustrated Lewis pair conditions
Dimesitylphosphinoisoprene reacts with a series of R-B(C6F5)2 boron Lewis acids by isomerization and subsequent 1,4-P/B addition to give the corresponding heterocyclic phosphonium/borate zwitterionic products.
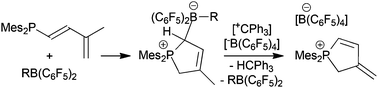
Dalton Trans., 2016,45, 6111-6117
https://doi.org/10.1039/C5DT03879K
Extending motifs in lithiocuprate chemistry: unexpected structural diversity in thiocyanate complexes
Lithio(thiocyanato)cuprates have been developed. These reveal planar, boat-shaped and chair-shaped Lipshutz-type dimers in the solid state, while Lipshutz-type and Gilman structures are seen in solution.

Dalton Trans., 2016,45, 6094-6104
https://doi.org/10.1039/C5DT03882K
Novel B(Ar′)2(Ar′′) hetero-tri(aryl)boranes: a systematic study of Lewis acidity
Nine homo- and hetero-tri(aryl)boranes related by stepwise aryl substitutions, are studied for H2 cleavage as part of an FLP, and comparative Lewis acidity/electrochemical measurements discussed.
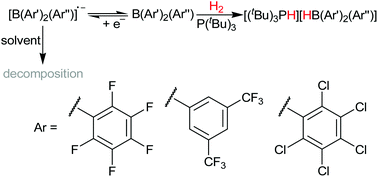
Dalton Trans., 2016,45, 6032-6043
https://doi.org/10.1039/C5DT03854E
Synthesis of 2-(lutidinyl)organoboranes and their reactivities against dihydrogen and pinacol borane
The reactivity of two 2,4,6-tris(trifluoromethyl)phenyl-substituted 2-(lutidinyl)organoboranes as intramolecular frustrated Lewis pairs was investigated.
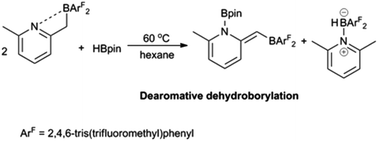
Dalton Trans., 2016,45, 6088-6093
https://doi.org/10.1039/C5DT03815D
Concise access to iminophosphonamide stabilized heteroleptic germylenes: chemical reactivity and structural investigation
A heteroleptic three coordinate germylene monochloride, its adduct with a Lewis acid, and germaacid-chloride & -ester derivatives with sulfur and selenium have been reported.
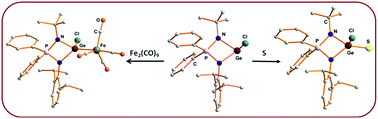
Dalton Trans., 2016,45, 6079-6087
https://doi.org/10.1039/C5DT02287H
Transition metal-mediated donor–acceptor coordination of low-oxidation state Group 14 element halides
The main group element triggered C–H bond activation of a Rh-bound Cp ligand is reported. The key aspect of this transformation is the presence of a highly Lewis acidic Group 14 element site.
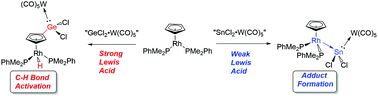
Dalton Trans., 2016,45, 6071-6078
https://doi.org/10.1039/C5DT03018H
The carboboration of Me3Si-substituted alkynes and allenes with boranes and borocations
ArylBCl2 and aryl and vinyl containing borocations synthesised by electrophilic borylation effect the carboboration of TMS-substituted alkynes and allenes.
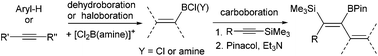
Dalton Trans., 2016,45, 6060-6070
https://doi.org/10.1039/C5DT03003J
The first examples of 1-D organic hybrid lanthanoid thioarsenates based on two [AsVS4]3− linkage modes
Two different [AsVS4]3− linkage modes first coexist in 1-D organic hybrid lanthanoid thioarsenates.
![Graphical abstract: The first examples of 1-D organic hybrid lanthanoid thioarsenates based on two [AsVS4]3− linkage modes](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C5DT02188J&imageInfo.ImageIdentifier.Year=2016)
Dalton Trans., 2016,45, 6015-6022
https://doi.org/10.1039/C5DT02188J
Salt metathesis versus protonolysis routes for the synthesis of silylamide Hauser base (R2NMgX; X = halogen) and amido-Grignard (R2NMgR) complexes
The effectiveness of simple synthetic routes to access silylamide Hauser base (R2NMgX; X = halogen) and amido-Grignard (R2NMgR) complexes from commercially available Grignard reagents is explored herein.
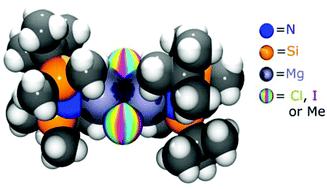
Dalton Trans., 2016,45, 6004-6014
https://doi.org/10.1039/C5DT02535D
Reduction of dichloro(diaza-phospha)stibanes – isolation of a donor-stabilized distibenium dication
A bulky NPN-substituted dichlorostibane was reduced with KC8 to afford a distibenium compound with a [Sb2]2+ ion which can be regarded as a dimerization product of a stiba-phospha-diazanediyl singlet biradicaloid. The intermediate formation of this singlet stiba-phospha-diazanediyl was proven by trapping experiments.
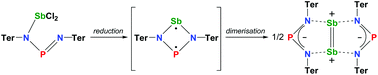
Dalton Trans., 2016,45, 6044-6052
https://doi.org/10.1039/C5DT02711J
Exploring structural and electronic effects in three isomers of tris{bis(trifluoromethyl)phenyl}borane: towards the combined electrochemical-frustrated Lewis pair activation of H2
Three B{C6H3(CF3)2}3 isomers have been studied, ortho-substituents quench FLP H2 cleavage via steric blocking and electron donation to the boron centre.
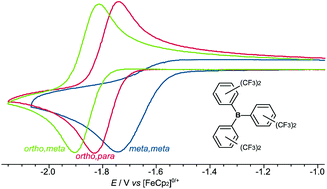
Dalton Trans., 2016,45, 6023-6031
https://doi.org/10.1039/C5DT01918D
Synthesis and characterization of bis(imino)pyridine complexes of divalent Mg and Zn
The synthesis and electronic structure of bis(imino)pyridine (I2P) complexes of the divalent metal ions, Zn(II) and Mg(II) are reported, and a correlation between the ligand Cim–Cpy bond lengths with the ligand torsion angle is described. Structural comparison with a new complex of Al(III) and previously reported Al(III) complexes is included.
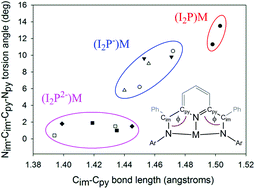
Dalton Trans., 2016,45, 5989-5998
https://doi.org/10.1039/C5DT01541C
Carbene insertion into a P–H bond: parent phosphinidene–carbene adducts from PH3 and bis(phosphinidene)mercury complexes
The insertion of an N-heterocyclic carbene into a P–H bond is the first step in a new synthesis of parent phosphinidene–carbene adducts from PH3. These adducts are used to generate bis(phosphinidene)mercury(II) complexes.
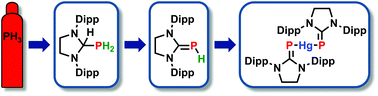
Dalton Trans., 2016,45, 5999-6003
https://doi.org/10.1039/C5DT01741F
Can main group systems act as superior catalysts for dihydrogen generation reactions? A computational investigation
The density functional theory (DFT) calculations reveal the potential of newly proposed main group germanium hydride systems to effect important chemical transformations, such as the catalytic cleavage of the O–H bond in water and alcohols, with significantly greater efficiency than the existing, state-of-the-art post-transition metal based systems.
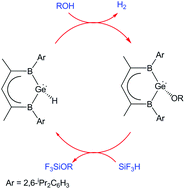
Dalton Trans., 2016,45, 5968-5977
https://doi.org/10.1039/C5DT01058F
Efficient synthetic methods for the installation of boron–nitrogen bonds in conjugated organic molecules
New synthetic methods for preparing gram quantities BN analogs of polycyclic aromatic hydrocarbons are highlighted. Such methods are key to proper evaluation of these materials in device applications.
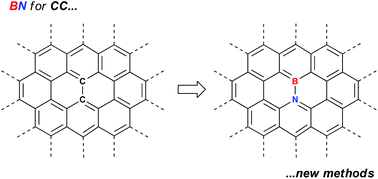
Dalton Trans., 2016,45, 5920-5924
https://doi.org/10.1039/C5DT03991F
About this collection
Guest edited by: Professor Doug Stephan, University of Toronto, Canada, and Dr Rebecca Melen, Cardiff University, UK.
This themed issue focusses on the field of main group chemistry, covering a diverse array of developments and potential applications in fields ranging from catalysis to molecular electronics. These have often evolved from studies in fundamental chemistry such as studies of the chemistry of low oxidation state and/or low coordination number compounds of the main group elements, fundamental studies in Lewis-acid/Lewis-base reactivity, main group heterocycles, free radicals and studies of the bonding in main group compounds.