Cyclic NHC-stabilized silylphosphinoalanes and -gallanes†
Abstract
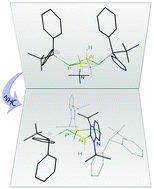
Four-membered Al/Ga–P ring systems were prepared by thermal hydrogen elimination between the primary silylphosphine H2PSitBuPh2 and the metal hydride precursors [(Me3N)MH3] (M = Al, Ga). The obtained cycles [(Me3N)HMP(SitBuPh2)]2 (M = Al: 1, Ga: 2) were functionalized by substituting the amine donor with the N-heterocyclic carbenes IPr and BImY to obtain the compounds [(NHC)HMP(SitBuPh2)]2 (IPr/Al: 3, BImY/Al: 4, IPr/Ga: 5, BImY/Ga: 6). All mentioned compounds feature an unusual external Lewis base stabilization as well as hydridic metal bound hydrogen atoms.
- This article is part of the themed collection: Main Group Transformations

 Please wait while we load your content...
Please wait while we load your content...