Pyridinium–phosphonium dications: highly electrophilic phosphorus-based Lewis acid catalysts†
Abstract
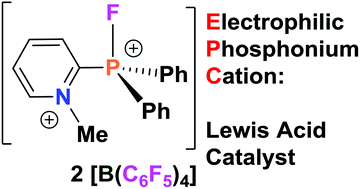
Using commercially available 2-pyridyldiphenylphosphine (o-NC5H4)PPh2, a family of electrophilic phosphonium cations [(o-NC5H4)PFPh2]+ (2) and dications [(o-MeNC5H4)PRPh2]2+ (R = F (4); Me (5)) were prepared. The Lewis acidity of these pyridinium–phosphonium dications was probed in Friedel–Crafts dimerization, hydrodefluorination, hydrosilylation, dehydrocoupling and hydrodeoxygenation reactions. The influence of the counterion on the catalytic activity of the electrophilic phosphonium cations is also discussed.
- This article is part of the themed collection: Main Group Transformations

 Please wait while we load your content...
Please wait while we load your content...