Efficient synthetic methods for the installation of boron–nitrogen bonds in conjugated organic molecules
Abstract
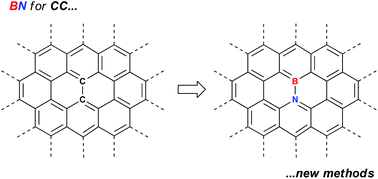
Polycyclic aromatic hydrocarbons in which one or more CC units have been replaced by isoelectronic BN units have attracted interest as potentially improved organic materials in various devices. This promise has been hampered by a lack of access to gram quantities of these materials. However, the exploitation of keystone reactions such as ring closing metathesis, borylative cyclization of amino styrenes and electrophilic borylation has lead to strategies for access to workable amounts of material. These strategies can be augmented by judicious postfunctionalization reactions to diversify the library of materials available. This Frontier article highlights some of the recent successes and shows that the long promised applications of BN-doped PAHs are beginning to be explored in a meaningful way.
- This article is part of the themed collection: Main Group Transformations

 Please wait while we load your content...
Please wait while we load your content...