Themed collection Interfacial Phenomena in (De)hydrogenation Reactions

Interfacial phenomena in (de)hydrogenation reactions
Editorial for the themed issue on interfacial phenomena in (de)hydrogenation reactions
Phys. Chem. Chem. Phys., 2013,15, 11985-11987
https://doi.org/10.1039/C3CP90089D
In situ/operando studies for the production of hydrogen through the water-gas shift on metal oxide catalysts
In situ/operando studies provide unique information about the active phase of catalysts used for hydrogen production through the water-gas shift reaction.
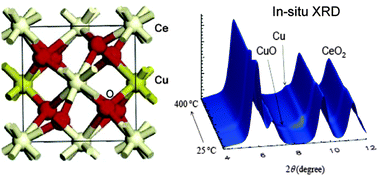
Phys. Chem. Chem. Phys., 2013,15, 12004-12025
https://doi.org/10.1039/C3CP50416F
Key unanswered questions about the mechanism of olefin hydrogenation catalysis by transition-metal surfaces: a surface-science perspective
A personal perspective is offered on the state of the art of our current understanding of the mechanism of olefin hydrogenations promoted by transition-metal catalysts.
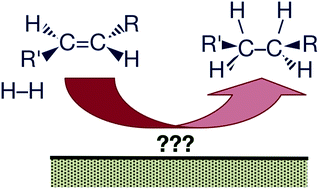
Phys. Chem. Chem. Phys., 2013,15, 11988-12003
https://doi.org/10.1039/C3CP50402F
Branched TiO2 nanoarrays sensitized with CdS quantum dots for highly efficient photoelectrochemical water splitting
This paper describes the design, characterization, and utilization of branched TiO2 nanoarrays sensitized with CdS quantum dots as anodes for photoelectrochemical water splitting.
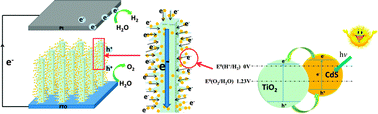
Phys. Chem. Chem. Phys., 2013,15, 12026-12032
https://doi.org/10.1039/C3CP51291F
Benzene adsorption on binary Pt3M alloys and surface alloys: a DFT study
Benzene adsorption does not change the segregation state of the catalyst, and d-band models predict benzene adsorption energy well.
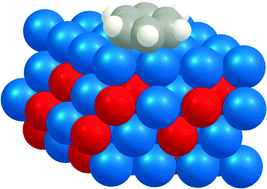
Phys. Chem. Chem. Phys., 2013,15, 12197-12214
https://doi.org/10.1039/C3CP50617G
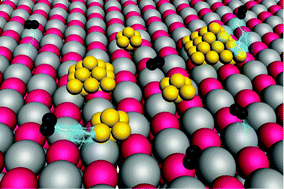
Single atom alloy surface analogs in Pd0.18Cu15 nanoparticles for selective hydrogenation reactions
We report a novel synthesis of nanoparticle Pd–Cu catalysts, containing only trace amounts of Pd, for selective hydrogenation reactions.
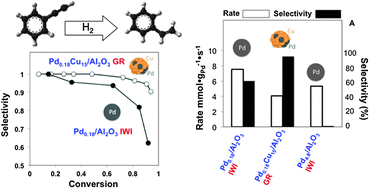
Phys. Chem. Chem. Phys., 2013,15, 12187-12196
https://doi.org/10.1039/C3CP51538A
Co-processing CH4 and oxygenates on Mo/H-ZSM-5: 2. CH4–CO2 and CH4–HCOOH mixtures
Co-processing CH4 and biomass-derived oxygenates in an attempt to couple CH4 dehydrogenation with biomass deoxygenation results in a staged bed with upstream reforming and downstream dehydroaromatization of CH4.
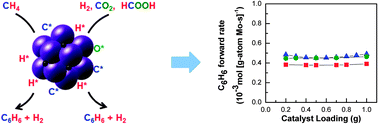
Phys. Chem. Chem. Phys., 2013,15, 12173-12179
https://doi.org/10.1039/C3CP50855B
Dehydration and dehydrogenation of ethylene glycol on rutile TiO2(110)
Temperature programmed desorption reveals a competition between ethylene glycol dehydration and dehydrogenation on TiO2(110).
Phys. Chem. Chem. Phys., 2013,15, 12180-12186
https://doi.org/10.1039/C3CP50687H
Reducing the dehydrogenation temperature of lithium hydride through alloying with germanium
Ge dramatically reduces the dehydrogenation temperature for LiH to just 270 °C through forming a series of lithium germanides.
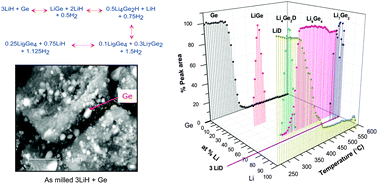
Phys. Chem. Chem. Phys., 2013,15, 12139-12146
https://doi.org/10.1039/C3CP51330K
In situ spectroscopic investigation of oxidative dehydrogenation and disproportionation of benzyl alcohol
Benzyl alcohol oxidation using supported AuPd alloy nanoparticles is investigated using in situ ATR-IR, DRIFT and inelastic neutron scattering spectroscopies.
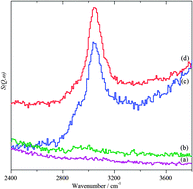
Phys. Chem. Chem. Phys., 2013,15, 12147-12155
https://doi.org/10.1039/C3CP50710F
Effect of oxide supports in stabilizing desirable Pt–Ni bimetallic structures for hydrogenation and reforming reactions
Comparative studies of the effect of oxide supports in stabilizing desirable Pt–Ni bimetallic structures show that the catalytic activity is PtNi/γ-Al2O3 ≫ PtNi/TiO2 for hydrogenation while PtNi/γ-Al2O3 < PtNi/TiO2 for reforming.
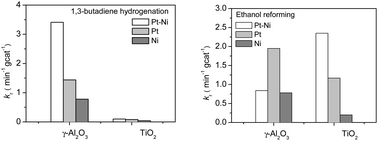
Phys. Chem. Chem. Phys., 2013,15, 12156-12164
https://doi.org/10.1039/C3CP44688C
Chemical transformations of glucose to value added products using Cu-based catalytic systems
Cu nanoparticles have been supported on aluminosilicate materials and succesfully employed as catalysts in the microwave-assisted conversion of glucose into valuable products via tandem formic acid-promoted dehydration (to 5-hydroxymethylfurfural—HMF) and further selective hydrogenation to 5-methylfurfuryl alcohol (MFA).
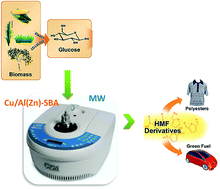
Phys. Chem. Chem. Phys., 2013,15, 12165-12172
https://doi.org/10.1039/C3CP50707F
Role of the surface–subsurface interlayer interaction in enhancing oxygen hydrogenation to water in Pd3Co alloy catalysts
Based on density functional theory calculations, we present mechanisms underlying the improvement in the catalytic performance of Pd-based alloys for oxygen hydrogenation to water by a thorough analysis of intra and interlayer orbital interactions.
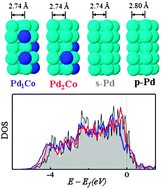
Phys. Chem. Chem. Phys., 2013,15, 12118-12123
https://doi.org/10.1039/C3CP50618E
Effects of O2 pressure on the oxidation of VOx/Pt(111)
‘High pressure’ is required to oxidize a vanadia thin film.
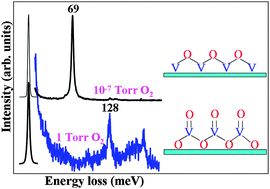
Phys. Chem. Chem. Phys., 2013,15, 12124-12131
https://doi.org/10.1039/C3CP50712B
Enhanced sulfur resistance of Ni/SiO2 catalyst for methanation via the plasma decomposition of nickel precursor
A Ni/SiO2 catalyst with fewer defects shows better sulfur resistance for CO methanation.
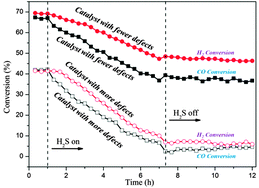
Phys. Chem. Chem. Phys., 2013,15, 12132-12138
https://doi.org/10.1039/C3CP50694K
Operando Raman spectroscopy study on the deactivation of Pt/Al2O3 and Pt–Sn/Al2O3 propane dehydrogenation catalysts
Operando Raman spectroscopy revealed that the addition of H2 alters the chemical nature of coke deposits on a Pt-based propane dehydrogenation catalyst.
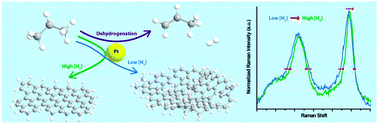
Phys. Chem. Chem. Phys., 2013,15, 12095-12103
https://doi.org/10.1039/C3CP50646K
A Langmuir–Hinshelwood approach to the kinetic modelling of catalytic ammonia decomposition in an integral reactor
Extended LHHW kinetic models are able to predict perfectly the performance of integral reactors during ammonia decomposition without considering any change of the mechanism of reaction.
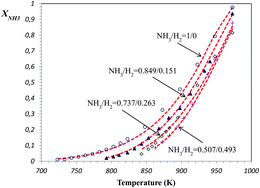
Phys. Chem. Chem. Phys., 2013,15, 12104-12117
https://doi.org/10.1039/C3CP50715G
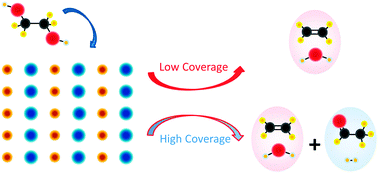
Selectivity in the initial C–H bond cleavage of n-butane on PdO(101)
Bond selectivity is coverage dependent for n-butane dissociation on PdO(101), with primary C–H bond cleavage strongly favored at low coverage.

Phys. Chem. Chem. Phys., 2013,15, 12075-12087
https://doi.org/10.1039/C3CP50659B
Fabrication of NiS modified CdS nanorod p–n junction photocatalysts with enhanced visible-light photocatalytic H2-production activity
NiS surface modified CdS nanorod p–n junction photocatalysts exhibit enhanced visible-light photocatalytic H2-production activity.
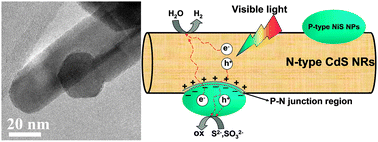
Phys. Chem. Chem. Phys., 2013,15, 12088-12094
https://doi.org/10.1039/C3CP50734C
Evolution of active catalysts for the selective oxidative dehydrogenation of methanol on Fe2O3 surface doped with Mo oxide
Build up Fe2(MoO4)3 with addition of MoO3 monolayers to Fe2O3. Ultimately, when ferric molybdate formation is saturated, a complete layer of molybdenum oxide forms at the surface.
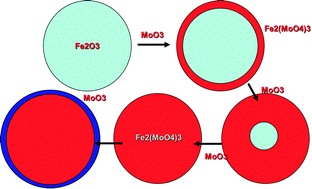
Phys. Chem. Chem. Phys., 2013,15, 12056-12067
https://doi.org/10.1039/C3CP50399B
Reaction mechanism of WGS and PROX reactions catalyzed by Pt/oxide catalysts revealed by an FeO(111)/Pt(111) inverse model catalyst
Water facilely dissociates on the Fe(II)CUS cations at the FeO(111)–Pt(111) interface to form hydroxyls that can react with CO(a) on the neighbouring Pt(111) sites to produce CO2 at low temperatures.
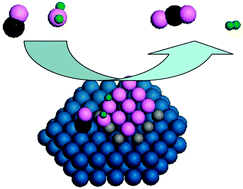
Phys. Chem. Chem. Phys., 2013,15, 12068-12074
https://doi.org/10.1039/C3CP50292A
The promotional effect of Sn-beta zeolites on platinum for the selective hydrogenation of α,β-unsaturated aldehydes
Pt impregnated on a Sn-beta catalyst results in a very promising catalyst for the selective hydrogenation of α,β-unsaturated aldehydes, compared to the PtSn co-impregnated beta catalysts.
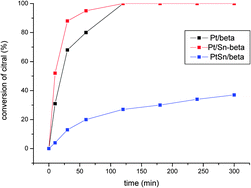
Phys. Chem. Chem. Phys., 2013,15, 12048-12055
https://doi.org/10.1039/C3CP50519G
New insight into the enhanced visible-light photocatalytic activities of B-, C- and B/C-doped anatase TiO2 by first-principles
The origin of visible-light absorption and photoactive enhancement for the B-, C- and B/C-doped TiO2 is investigated by first-principles.
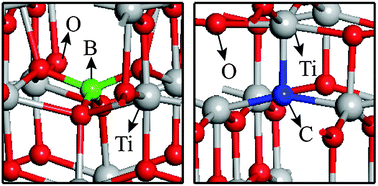
Phys. Chem. Chem. Phys., 2013,15, 12040-12047
https://doi.org/10.1039/C3CP44651D
Enhanced photocatalytic H2-production activity of TiO2 using Ni(NO3)2 as an additive
Photocatalytic H2 production using TiO2 was demonstrated using Ni(NO3)2 as an additive, giving a H2-production rate of 2547 μmol h−1 g−1 with a quantum efficiency of 8.1%.

Phys. Chem. Chem. Phys., 2013,15, 12033-12039
https://doi.org/10.1039/C2CP43628K
About this collection
The Interfacial Phenomena in (De)hydrogenation Reactions themed issue investigates interfacial phenomena at the molecular/atomic level. Guest edited by Jinlong Gong.