Vanadium oxide (VOx) has been extensively used in many oxidation and selective oxidation reactions. In this study, VOx thin films were prepared in an ultra-high vacuum (UHV) chamber by evaporating V onto a Pt(111) surface followed by subsequent oxidation at 623 K in 1 × 10−7 Torr O2, and further oxidized in the ‘high-pressure’ reaction cell with 1 Torr O2. The film quality and structure were investigated by high-resolution electron energy loss spectroscopy (HREELS), X-ray photoelectron spectroscopy (XPS), low energy electron diffraction (LEED), low energy ion scattering spectroscopy (LEIS), Auger electron spectroscopy (AES), and in situ infrared reflection absorption spectroscopy (IRAS). On the Pt(111) surface, VOx forms isolated O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) VOx (x = 0–3) species, surface two-dimensional (2D) (2 × 2)-V2O3 domains, a bi-layer structure with a (3√3 × 6) arrangement, and a complicated tri-layer structure as the coverage increases from submonolayer to multilayer. Under the UHV conditions, the oxidation state of V is mainly +3 and the stability was found to be surface V2O3 > bi-layer V2O3 > tri-layer one. After exposing to 0.3–1 Torr O2, VOx can be oxidized to higher oxidation states, mainly V2O5, as evidenced by the shifts of the core-level binding energies and presence of V
VOx (x = 0–3) species, surface two-dimensional (2D) (2 × 2)-V2O3 domains, a bi-layer structure with a (3√3 × 6) arrangement, and a complicated tri-layer structure as the coverage increases from submonolayer to multilayer. Under the UHV conditions, the oxidation state of V is mainly +3 and the stability was found to be surface V2O3 > bi-layer V2O3 > tri-layer one. After exposing to 0.3–1 Torr O2, VOx can be oxidized to higher oxidation states, mainly V2O5, as evidenced by the shifts of the core-level binding energies and presence of V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. These results indicate that thorough oxidation of VOx requires sufficiently high O2 pressure, and that vanadium-based catalysts may possess higher oxidation states under most reaction conditions in the presence of O2.
O. These results indicate that thorough oxidation of VOx requires sufficiently high O2 pressure, and that vanadium-based catalysts may possess higher oxidation states under most reaction conditions in the presence of O2.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) VOx (x = 0–3) species, surface two-dimensional (2D) (2 × 2)-V2O3 domains, a bi-layer structure with a (3√3 × 6) arrangement, and a complicated tri-layer structure as the coverage increases from submonolayer to multilayer. Under the UHV conditions, the oxidation state of V is mainly +3 and the stability was found to be surface V2O3 > bi-layer V2O3 > tri-layer one. After exposing to 0.3–1 Torr O2, VOx can be oxidized to higher
VOx (x = 0–3) species, surface two-dimensional (2D) (2 × 2)-V2O3 domains, a bi-layer structure with a (3√3 × 6) arrangement, and a complicated tri-layer structure as the coverage increases from submonolayer to multilayer. Under the UHV conditions, the oxidation state of V is mainly +3 and the stability was found to be surface V2O3 > bi-layer V2O3 > tri-layer one. After exposing to 0.3–1 Torr O2, VOx can be oxidized to higher ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. These results indicate that thorough
O. These results indicate that thorough 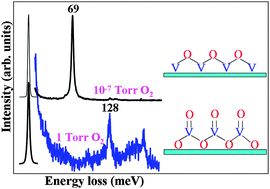

 Please wait while we load your content...
Please wait while we load your content...