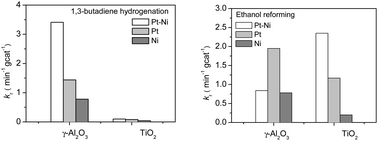
Previous surface science studies have shown that bimetallic surfaces often show unique activity for reactions involving the consumption and production of hydrogen, such as hydrogenation and reforming reactions, respectively. These two types of reactions require different bimetallic configurations. For example, for the Pt–Ni bimetallic system, the desirable structure is Pt-terminated for hydrogenation while Ni-terminated for reforming. In the current study, 1,3-butadiene hydrogenation and ethanol reforming were used as probe reactions to investigate the effect of oxide supports (γ-Al2O3 and TiO2) on the structural and catalytic properties of Pt–Ni catalysts. The supported catalysts were characterized by transmission electron microscopy (TEM) and extended X-ray absorption fine structure (EXAFS). The reactions were carried out in a batch reactor equipped with a Fourier transform infrared (FTIR) spectrometer. For ethanol reforming, Pt–Ni/TiO2 showed higher activity than Pt–Ni/γ-Al2O3, and the Pt–Ni bimetallic catalyst outperformed the monometallic catalysts on TiO2 but not on γ-Al2O3. In contrast, for 1,3-butadiene hydrogenation, Pt–Ni/TiO2 showed much lower activity than Pt–Ni/γ-Al2O3. Density functional theory (DFT) calculations of Pt–Ni nanoparticles on γ-Al2O3 and TiO2 were performed to provide possible explanations for the different modification effects of the two oxide supports.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...