Themed collection Functionalization of unreactive C-H bonds

Cyclometallated complexes as catalysts for C–H activation and functionalization
This highlight describes an emerging trend in the C–H activation field: the use of the cyclometallated catalysts for the challenging and unprecedented direct transformations.
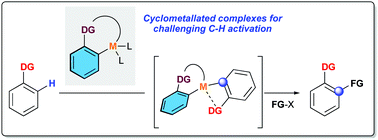
Chem. Commun., 2022,58, 483-490
https://doi.org/10.1039/D1CC05195D
Pd-catalyzed bidentate auxiliary assisted remote C(sp3)–H functionalization
This article presents “state of art” trends in achieving the bidentate auxiliary assisted distal sp3 C–H functionalization beyond proximal sites. The substrate scope and mechanistic underpinnings of the key methodologies are highlighted.
Chem. Commun., 2021,57, 13221-13233
https://doi.org/10.1039/D1CC05291H
Visible light-induced functionalization of indazole and pyrazole: a recent update
This review systematically discusses all of the visible light-induced functionalization methodologies and mechanisms of two significant heterocycles, indazole and pyrazole.
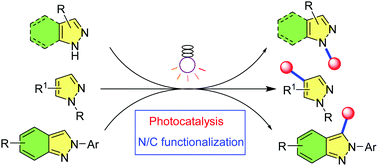
Chem. Commun., 2022,58, 4435-4455
https://doi.org/10.1039/D2CC00002D
Looking deep into C–H functionalization: the synthesis and application of cyclopentadienyl and related metal catalysts
This feature review is focused on recent key applications of commonly used transition-metal Cp-type catalysts for C–H bond functionalizations.
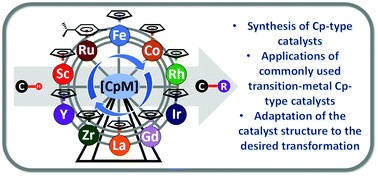
Chem. Commun., 2022,58, 3101-3121
https://doi.org/10.1039/D1CC07040A
Diastereoselective palladium-catalyzed functionalization of prochiral C(sp3)–H bonds of aliphatic and alicyclic compounds
Advancements in the palladium-catalyzed functionalization of diastereotopic or prochiral C(sp3)–H bonds generating stereogenic centers and stereo-arrays in aliphatic compounds have been highlighted.
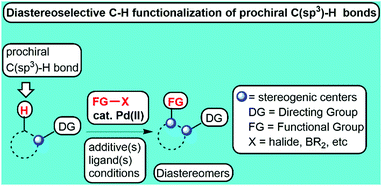
Chem. Commun., 2022,58, 2612-2633
https://doi.org/10.1039/D1CC05649B
Advances in allylic and benzylic C–H bond functionalization enabled by metallaphotoredox catalysis
Merging two powerful methodologies, metallaphoto-catalyzed benzylic and allylic C(sp3)–H bond functionalizations provide a series of general and mild approaches for diversification of alkylbenzenes and alkenes.

Chem. Commun., 2022,58, 171-184
https://doi.org/10.1039/D1CC06285A
Recent advances on non-precious metal-catalyzed C–H functionalization of N-heteroarenes
Recent advances on non-precious metal-catalyzed C–H functionalization of N-heteroarenes are discussed.

Chem. Commun., 2022,58, 10-28
https://doi.org/10.1039/D1CC05899A
Chemically robust and readily available quinoline-based PNN iron complexes: application in C–H borylation of arenes
Synthesis of air-stable PNN iron pincer complexes and their application in the C–H borylation of arenes.

Chem. Commun., 2021,57, 13246-13258
https://doi.org/10.1039/D1CC04877E
Recent progress in the oxidative coupling of unactivated Csp3–H bonds with other C–H bonds
The oxidative coupling between two carbon–hydrogen (C–H) bonds offers the most straightforward pathway to construct C–C bonds from hydrocarbons without pre-functionalization, exhibiting high step- and atom-economy.

Chem. Commun., 2021,57, 13288-13296
https://doi.org/10.1039/D1CC04802C
Ir-catalyzed proximal and distal C–H borylation of arenes
Over the past two decades, the C–H bond activation and functionalization reaction has been known as a prevailing method for the construction of carbon–carbon and carbon–heteroatom bonds using various transition metal catalysts.
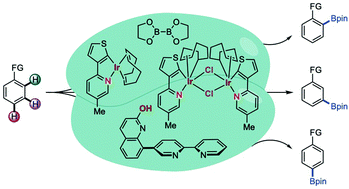
Chem. Commun., 2021,57, 13059-13074
https://doi.org/10.1039/D1CC05104K
Pd-Catalyzed and ligand-enabled alkene difunctionalization via unactivated C–H bond functionalization
Pd-Catalyzed and ligand-enabled difunctionalization of olefins through the unreactive C–H bond functionalization of either alkene or their respective coupling partners was summarized.
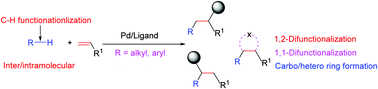
Chem. Commun., 2021,57, 12045-12057
https://doi.org/10.1039/D1CC04397H
Transition metal catalyzed C–H bond activation by exo-metallacycle intermediates
exo-Metallacycles have become the key reaction intermediates in activating various remote C(sp2)–H and C(sp3)–H bonds in the past decade and aided in achieving unusual site-selectivity.
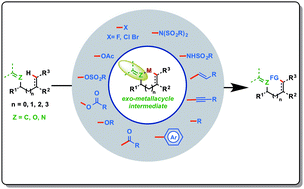
Chem. Commun., 2021,57, 11885-11903
https://doi.org/10.1039/D1CC05042G
Photocatalytic functionalizations of alkynes
Visible light mediated functionalizations have significantly expanded the scope of alkynes by unraveling new mechanistic pathways and enabling their transformation to diverse structural entities.
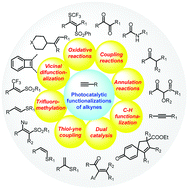
Chem. Commun., 2021,57, 11285-11300
https://doi.org/10.1039/D1CC04014F
Chain-walking reactions of transition metals for remote C–H bond functionalization of olefinic substrates
Transition metal-assisted remote C–H bond activation at the non-classical reaction sites of various olefinic substrates with the aid of a chain-walking process is depicted in this feature article.
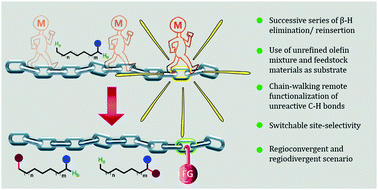
Chem. Commun., 2021,57, 11110-11130
https://doi.org/10.1039/D1CC04370F
C–H bond functionalization by high-valent cobalt catalysis: current progress, challenges and future perspectives
Over the last decade, high-valent cobalt catalysis has earned a place in the spotlight as a valuable tool for C–H activation and functionalization.
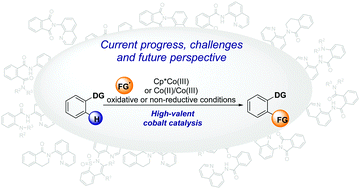
Chem. Commun., 2021,57, 10827-10841
https://doi.org/10.1039/D1CC04382J
Homogeneous catalytic C(sp3)–H functionalization of gaseous alkanes
The conversion of light alkanes into bulk chemicals is becoming an important challenge as it effectively avoids the use of prefunctionalized alkylating reagents.
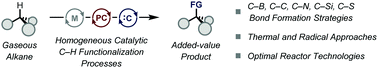
Chem. Commun., 2021,57, 9956-9967
https://doi.org/10.1039/D1CC04073A
Native carboxyl group-assisted C–H acetoxylation of hydrocinnamic and phenylacetic acids
Exogenous-directing-group free C–H acetoxylation of hydrocinnamic and phenylacetic acid derivatives is reported.

Chem. Commun., 2022,58, 4993-4996
https://doi.org/10.1039/D2CC00459C
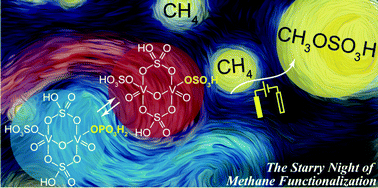
Bisulfate as a redox-active ligand in vanadium-based electrocatalysis for CH4 functionalization
The bisulfate ligand within a reported vanadium (V) electrocatalyst serves as the active site for CH4 functionalization. Activity decreases if the bisulfate ligand is substituted with a redox-inactive dihydrogen phosphate.
Chem. Commun., 2022,58, 2524-2527
https://doi.org/10.1039/D1CC06596C
Cobalt-catalyzed highly diastereoselective [3 + 2] carboannulation reactions: facile access to substituted indane derivatives
Cobalt(II)-catalyzed oxidative annulation of hydrazones with heterobicyclic alkenes has been accomplished for accessing functionalized indane derivatives under aerobic conditions.
![Graphical abstract: Cobalt-catalyzed highly diastereoselective [3 + 2] carboannulation reactions: facile access to substituted indane derivatives](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1CC05245D&imageInfo.ImageIdentifier.Year=2022)
Chem. Commun., 2022,58, 1386-1389
https://doi.org/10.1039/D1CC05245D
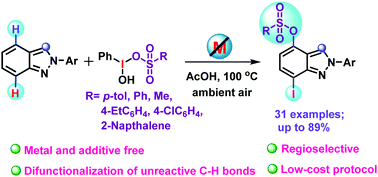
Remote difunctionalization of 2H-indazoles using Koser's reagents
We report a regioselective remote difunctionalization of unreactive C–H bonds of 2H-indazoles with Koser's reagents to provide C-4,7 substituted 2H-indazole derivatives.
Chem. Commun., 2022,58, 981-984
https://doi.org/10.1039/D1CC06129A
Palladium-catalyzed oxidative C–H/C–H cross-coupling of pyrazolo[1,5-a]azines with five-membered heteroarenes
The use of Pd(OAc)2 catalyst and AgOAc oxidant enabled the direct regioselective oxidative C–H/C–H cross-coupling of pyrazolo[1,5-a]azineswith various five-membered heteroarenes without the need of pre-activation and/or directing groups.
![Graphical abstract: Palladium-catalyzed oxidative C–H/C–H cross-coupling of pyrazolo[1,5-a]azines with five-membered heteroarenes](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1CC06337E&imageInfo.ImageIdentifier.Year=2022)
Chem. Commun., 2022,58, 827-830
https://doi.org/10.1039/D1CC06337E
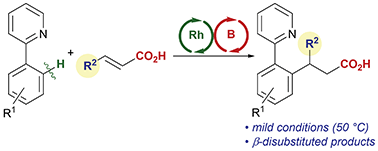
Cp*Rh(III)/boron hybrid catalysis for directed C–H addition to β-substituted α,β-unsaturated carboxylic acids
The C–H bond addition reaction of 2-phenylpyridine derivatives with α,β-unsaturated carboxylic acids catalyzed by Cp*Rh(III)/BH3·SMe2 is reported.
Chem. Commun., 2022,58, 76-79
https://doi.org/10.1039/D1CC05956D
Contrasteric coupling of allenes and tetrahydroisoquinolines by iron-catalysed allenic C(sp2)–H functionalisation
An iron-catalysed C–H functionalisation of simple monosubstituted allenes for the synthesis of 1-tetrahydroisoquinolinyl 1,1-disubstituted allenes is reported.

Chem. Commun., 2021,57, 13329-13332
https://doi.org/10.1039/D1CC05949A
An organophotoredox-catalyzed redox-neutral cascade involving N-(acyloxy)phthalimides and allenamides: synthesis of indoles
An efficient visible organophotoredox-catalyzed redox-neutral radical cascade involving allenamides and alkyl N-(acyloxy)phthalimides allowing the synthesis of biologically important indole scaffolds is achieved.

Chem. Commun., 2021,57, 13130-13133
https://doi.org/10.1039/D1CC05397C
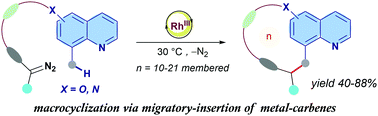
Rh(III)-Catalyzed mild straightforward synthesis of quinoline-braced cyclophane macrocycles via migratory insertion
A mild and straightforward Rh(III)-catalysed macrocylization strategy is developed to deliver quinoline braced cyclophane type macrocycles based on migratory insertion of metal–carbenes into the C8-methyl group of quinoline scaffolds.
Chem. Commun., 2021,57, 13134-13137
https://doi.org/10.1039/D1CC04418D
Intermediates and mechanism in iron-catalyzed C–H methylation with trimethylaluminum
Iron-catalyzed C–H methylation with organoaluminum reagents accesses reactive cyclometalated iron(II) intermediates.
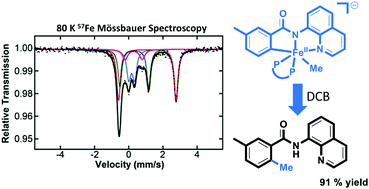
Chem. Commun., 2021,57, 12784-12787
https://doi.org/10.1039/D1CC05607G
Room-temperature C–H bond alkynylation by merging cobalt and photocatalysts
A new protocol is developed for the mono- and bis-ortho-C–H alkynylation of easily accessible benzamide derivatives using alkynyl bromides at room temperature by merging cobalt and photocatalysts.
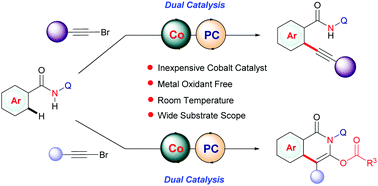
Chem. Commun., 2021,57, 12167-12170
https://doi.org/10.1039/D1CC05263B
Iridium-catalyzed enantioselective addition of an N-methyl C–H bond to α-trifluoromethylstyrenes via C–H activation
The Ir-catalyzed enantioselective addition of an N-methyl C–H bond of 2-(methylamino)pyridine derivatives to α-trifluoromethylstyrenes proceeded via C–H activation to give chiral γ-branched amines having a trifluoromethyl-substituted stereocenter.
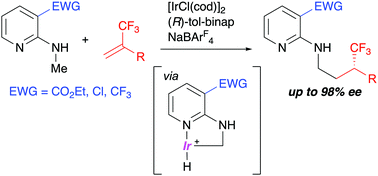
Chem. Commun., 2021,57, 11787-11790
https://doi.org/10.1039/D1CC05076A
Sterically controlled C–H alkenylation of pyrroles and thiophenes
Pd-catalyzed aerobic C–H alkenylations at the unhindered positions of N-alkylpyrroles and 3-substituted thiophenes are developed using a pyrazolonaphthyridine ligand.
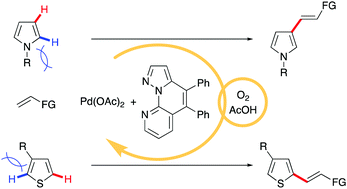
Chem. Commun., 2021,57, 11791-11794
https://doi.org/10.1039/D1CC04378A
Co(III)-catalysed regioselective linear C(8)–H olefination of isoquinolone with terminal aromatic and aliphatic alkynes
A regioselective C8 linear olefination of isoquinoline-1H-2-one with terminal (aromatic and aliphatic) alkynes is reported under Co(III) catalysis.
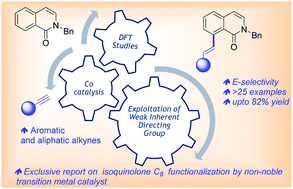
Chem. Commun., 2021,57, 11613-11616
https://doi.org/10.1039/D1CC04541E
Regiodivergent CDC reactions of aromatic aldehydes with unactivated arenes controlled by transient directing strategy
It was found for the first time that transient directing group controlled regiodivergent CDC reactions at both sp2 and sp3 hybridized carbons of aromatic compounds with palladium catalyst.

Chem. Commun., 2021,57, 11229-11232
https://doi.org/10.1039/D1CC04121E
Visible-light-promoted 3,5-dimethoxyphenyl glycoside activation and glycosylation
A new glycosylation method promoted by visible light with 3,5-dimethoxyphenyl glycoside as the donor was developed.
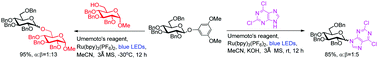
Chem. Commun., 2021,57, 10899-10902
https://doi.org/10.1039/D1CC04473G
Easy access to secondary and tertiary alcohols via metal-free and light mediated radical carbonyl allylation
An organo-photocatalyst catalyzed allylation of aryl ketones and aldehydes using readily available unactivated allylic substrates has been reported.
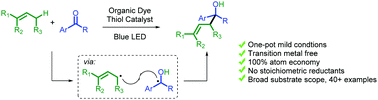
Chem. Commun., 2021,57, 10783-10786
https://doi.org/10.1039/D1CC04585G
A soluble iron(II)-phthalocyanine-catalyzed intramolecular C(sp3)–H amination with alkyl azides
A soluble iron(II)-phthalocyanine, [FeII(tBu4Pc)(py)2], is an effective catalyst in intramolecular C(sp3)–H bond amination of alkyl azides to give the amination products in moderate to excellent yields with a broad substrate scope.
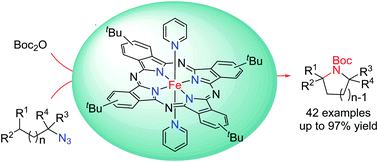
Chem. Commun., 2021,57, 10711-10714
https://doi.org/10.1039/D1CC04573C
Enantioselective first total syntheses of the antiviral natural products xiamycins D and E
The enantioselective first total syntheses of marine pentacyclic indolosesquiterpenoids xiamycins D (4) and E (5) have been described for the first time to the best of our knowledge.
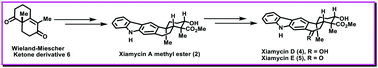
Chem. Commun., 2021,57, 10644-10646
https://doi.org/10.1039/D1CC04739F
Ruthenium(II)-catalyzed C–H activation and (4+2) annulation of aromatic hydroxamic acid esters with allylic amides
A (4+2) annulation under Ru(II)-catalysis is reported using aromatic hydroxamic acid esters as the oxidizing directing group and allylic amides as unactivated olefin coupling partners, delivering a wide variety of aminomethyl isoquinolinones in good to excellent yields.
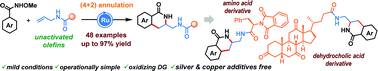
Chem. Commun., 2021,57, 10536-10539
https://doi.org/10.1039/D1CC04422B
Synthesis of axially chiral biaryl thioglycosides through thiosugar-directed Pd-catalyzed asymmetric C–H activation
An atroposelective Fujiwara–Moritani reaction towards the synthesis of alkenylthioglycoside is reported. β-thioglucose moiety is used both as a directing group for the C–H bond activation and as a chiral source inducing axial chirality.
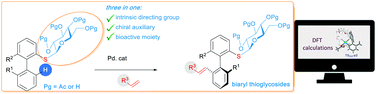
Chem. Commun., 2021,57, 10355-10358
https://doi.org/10.1039/D1CC03971G
Tris-NHC-propagated self-supported polymer-based Pd catalysts for heterogeneous C–H functionalization
Three-dimensionally propagated imidazolium-containing mesoporous coordination polymer and organic polymer platforms were assembled to develop the demanding single-site heterogeneous ‘Pd(II)–NHC’-based oxidative C–H functionalization catalysts.

Chem. Commun., 2021,57, 10182-10185
https://doi.org/10.1039/D1CC04429J
K2S2O8 activation by glucose at room temperature for the synthesis and functionalization of heterocycles in water
A diverse functionalization and synthesis of heterocycles using K2S2O8 as oxidant and glucose as persulfate activator in water at room temperature is reported.
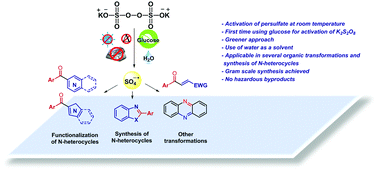
Chem. Commun., 2021,57, 8437-8440
https://doi.org/10.1039/D1CC03777C
Rhodium-catalysed direct formylmethylation using vinylene carbonate and sequential dehydrogenative esterification
A rhodium-catalysed direct formylmethylation adopting vinylene carbonate as an ethynol equivalent is reported.
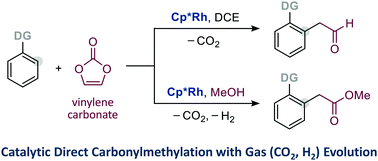
Chem. Commun., 2021,57, 8280-8283
https://doi.org/10.1039/D1CC03362J
Easy access to drug building-blocks through benzylic C–H functionalization of phenolic ethers by photoredox catalysis
A visible light-mediated photocatalyzed C–C-bond benzylic functionalization of phenolic ether-containing drug-like compounds is presented.
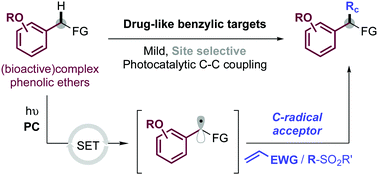
Chem. Commun., 2021,57, 6756-6759
https://doi.org/10.1039/D1CC01756J
Z-Selective Pd-catalyzed 2,2,2-trifluoroethylation of acrylamides at room temperature
A straightforward methodology for the selective synthesis of high-value added Z-2,2,2-trifluoroethylated acrylamides by Pd-catalyzed C–H bond activation at room temperature.
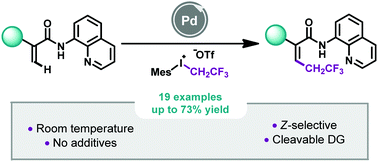
Chem. Commun., 2021,57, 6241-6244
https://doi.org/10.1039/D1CC02007B
Catalyst-controlled site-selective N–H and C3-arylation of carbazole via carbene transfer reactions
A site-selective direct arylation reaction of carbazole and other N-heterocycles with diazo-naphthalen-2(1H)-ones has been developed.
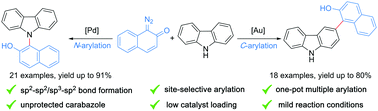
Chem. Commun., 2021,57, 6193-6196
https://doi.org/10.1039/D1CC01863A
Pd(II)-Catalyzed enantioselective arylation of unbiased methylene C(sp3)–H bonds enabled by a 3,3′-F2-BINOL ligand
Pd(II)-Catalyzed highly enantioselective arylation of unbiased methylene C(sp3)–H bonds using easily accessible 3,3′-F2-BINOL as a ligand was reported.
Chem. Commun., 2021,57, 5562-5565
https://doi.org/10.1039/D1CC01690C
About this collection
The functionalization of unreactive carbon-hydrogen (C–H) bonds has evolved as an atom- and step-economical, environmentally benign strategy in organic synthesis. This themed collection deals with a wide range of strategies regarding the functionalization of unreactive C–H bonds by world-leading researchers in this field. The principal purpose of this collection is to illustrate the diversity of research on the functionalization of unreactive C–H bonds. Therefore, this special collection, Guest Edited by Tatiana Besset (University of Rouen), Fumitoshi Kakiuchi (Keio University), and Debabrata Maiti (Indian Institute of Technology-Bombay) includes: the development of new useful methodologies using base metal catalysts; various syntheses of functionalized compounds utilized in medicinal and materials chemistry; the utilization of renewable feedstock’s; employing electronic or optical energies for generating reactive intermediates; and synthetic processes towards industrial usages that have opened a new era for organic synthesis.