Cp*Rh(iii)/boron hybrid catalysis for directed C–H addition to β-substituted α,β-unsaturated carboxylic acids†
Abstract
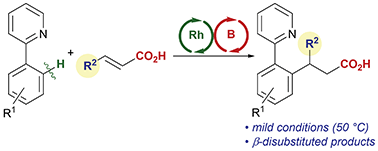
The C–H bond addition reaction of 2-phenylpyridine derivatives with α,β-unsaturated carboxylic acids catalyzed by Cp*Rh(III)/BH3·SMe2 is reported. Activation of C–H bonds with the rhodium catalyst and activation of α,β-unsaturated carboxylic acids with the boron catalyst cooperatively work, and a BINOL-urea hybrid ligand significantly improved the reactivity. With the optimized hybrid catalytic system, various β-disubstituted carboxylic acids were obtained under mild reaction conditions.
- This article is part of the themed collection: Functionalization of unreactive C-H bonds


 Please wait while we load your content...
Please wait while we load your content...