Z-Selective Pd-catalyzed 2,2,2-trifluoroethylation of acrylamides at room temperature†
Abstract
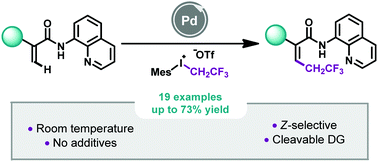
A straightforward 2,2,2-trifluoroethylation of acrylamides by Pd-catalyzed C–H bond activation was reported by using a fluorinated hypervalent iodine reagent as a coupling partner. At room temperature, this additive-free approach allowed the synthesis of Z-2,2,2-trifluoroethylated acrylamides (19 examples, up to 73% yield) in a stereoselective manner. Under these mild reaction conditions, the methodology turned out to be functional group tolerant and mechanistic studies gave us a better understanding of the transformation.
- This article is part of the themed collection: Functionalization of unreactive C-H bonds


 Please wait while we load your content...
Please wait while we load your content...