Themed collection Elegant Synthetic Routes to Indole Derivatives

Remote stereocontrolled asymmetric 1,6-addition/1,4-addition cascade reactions between cyclic dienones and 2-indolinethiones
Enantioselective 1,6-addition/1,4-addition cascade reactions between cyclic dienones and 2-indolnethiones have been realized, affording chiral spiro[thiopyranoindole-cyclohexanone] scaffolds facilely and efficiently.

RSC Adv., 2016,6, 106676-106679
https://doi.org/10.1039/C6RA19916J
Cu-Catalyzed ligand-free synthesis of rosuvastatin based novel indole derivatives as potential anticancer agents
Rosuvastatin based indoles showed anti-proliferative and apoptotic activities and an increase of p21 mRNA expression levels in zebrafish larvae.

RSC Adv., 2016,6, 100487-100493
https://doi.org/10.1039/C6RA20148B
Scandium triflate-catalyzed selective ring opening and rearrangement reaction of spiro-epoxyoxindole and carbonyl compounds
Scandium triflate-catalyzed reactions between spiro-epoxyoxindole and carbonyl compounds have been disclosed. Furthermore, an unprecedented rearrangement takes place to yield alkylidene oxindoles when aromatic aldehydes are used as carbonyl components.
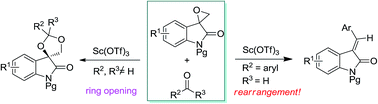
RSC Adv., 2016,6, 100307-100311
https://doi.org/10.1039/C6RA21264F
Cu-catalyzed β-functionalization of saturated ketones with indoles: a one-step synthesis of C3-substituted indoles
One-pot synthesis of β-indolylketones from saturated ketones and indoles was reported, which was useful in the synthesis of numerous heterocycles.
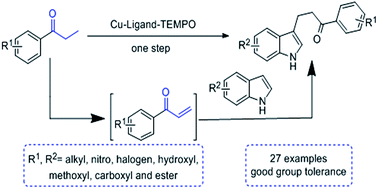
RSC Adv., 2016,6, 89181-89184
https://doi.org/10.1039/C6RA19000F
NBS-mediated dinitrogen extrusion of diazoacetamides under catalyst-free conditions: practical access to 3-bromooxindole derivatives
A synthetically useful reaction via NBS-mediated dinitrogen extrusion of N-aryl diazoacetamides under catalyst-free conditions is disclosed, which gives the 3-bromooxindoles in high to excellent yields with high selectivity via a non carbene process.
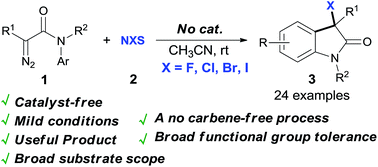
RSC Adv., 2016,6, 70221-70225
https://doi.org/10.1039/C6RA16868J
Concise approach to pyrrolizino[1,2-b]indoles from indole-derived donor–acceptor cyclopropanes
A direct approach to previously unexplored pyrrolizidino[1,2-b]indole system from indole-derived donor–acceptor cyclopropanes based on a sequence of three synthetic steps was developed.
![Graphical abstract: Concise approach to pyrrolizino[1,2-b]indoles from indole-derived donor–acceptor cyclopropanes](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6RA11233A&imageInfo.ImageIdentifier.Year=2016)
RSC Adv., 2016,6, 62014-62018
https://doi.org/10.1039/C6RA11233A
Gold-catalyzed formation of indole derivatives from 2-alkynyl arylazides and oxygen-containing heterocycles
Gold-catalyzed ring-opening reactions of oxygen-containing heterocycles.
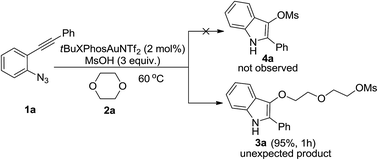
RSC Adv., 2016,6, 56319-56322
https://doi.org/10.1039/C6RA09761H
AlCl3-mediated heteroarylation-cyclization strategy: one-pot synthesis of fused quinoxalines containing the central core of Lamellarin D
Pyrano[3,4-b]indole fused quinoxalines were synthesized via an AlCl3-mediated heteroarylation-cyclization method as potential anticancer agents.
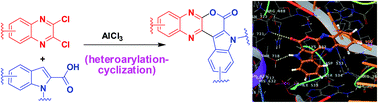
RSC Adv., 2016,6, 48324-48328
https://doi.org/10.1039/C6RA07507J
Tandem Mannich/Diels–Alder reactions for the synthesis of indole compound libraries
A core scaffold for screening library production was synthesized in just four steps using a tandem Mannich/Diels–Alder sequence.
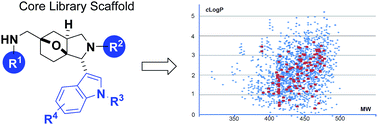
RSC Adv., 2016,6, 46654-46657
https://doi.org/10.1039/C6RA08786H
The dicarbonylation of indoles via Friedel–Crafts reaction with dicarbonyl nitrile generated in situ and retro-cyanohydrination
The reaction of indole and β-carbonyl nitrile to generate dicarbonyl indoles has been developed. This process involves α-oxonation of the β-carbonyl nitrile, Friedel–Crafts reaction with indoles and retro-cyanohydrination form dicarbonyl indoles.

RSC Adv., 2016,6, 44029-44033
https://doi.org/10.1039/C6RA04016K
Direct and site-selective Pd(II)-catalyzed C-7 arylation of indolines with arylsilanes
The palladium-catalyzed oxidative arylation of indolines with arylsilanes at the C-7 position via C–H bond activation has been reported.
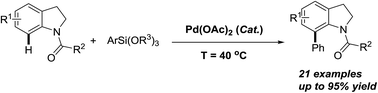
RSC Adv., 2016,6, 39292-39295
https://doi.org/10.1039/C6RA06915K
Rhodium(II)-catalyzed intramolecular annulation of 1-sulfonyl-1,2,3-triazoles with indoles: facile synthesis of functionalized tetrahydro-β-carbolines
A novel rhodium(II)-catalyzed intramolecular annulation of 1-sulfonyl-1,2,3-triazoles with indoles has been developed, which enables the facile synthesis of various 4-functionalized tetrahydro-β-carbolines and relevant scaffolds from readily available starting materials.

RSC Adv., 2016,6, 30835-30839
https://doi.org/10.1039/C6RA02923J
Mg(ClO4)2-promoted [4 + 3] cycloaddition of oxindole derivatives with conjugated dienes: concise synthesis of spirocycloheptane oxindole derivatives
Novel Mg(ClO4)2-promoted [4 + 3] cycloaddition reaction of oxindole derivatives and cyclopentadiene was achieved for the construction of the spirocycloheptane oxindole skeleton.
![Graphical abstract: Mg(ClO4)2-promoted [4 + 3] cycloaddition of oxindole derivatives with conjugated dienes: concise synthesis of spirocycloheptane oxindole derivatives](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6RA03259A&imageInfo.ImageIdentifier.Year=2016)
RSC Adv., 2016,6, 26954-26958
https://doi.org/10.1039/C6RA03259A
Synthesis of novel fluorescent 12a-aryl substituted indoxylisoquinolines via aryne-induced domino process
A novel effective protocol towards indolo[2,1-a]isoquinolinones via aryne-induced migration of aryl-anion in aryloxy substituted 3,4-dihydroisoquinolines is reported.
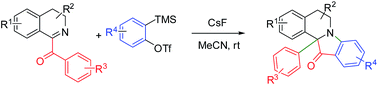
RSC Adv., 2016,6, 12642-12646
https://doi.org/10.1039/C5RA25323C
PIFA-mediated selenylative spirocyclization of indolyl ynones: facile access to selenated spiro[cyclopentenone-1,3′-indoles]
A fast selenylative spirocyclization of indolyl ynones mediated by PIFA has been developed. This transformation was enabled by the reactive RSeOCOCF3 species generated in situ from diselenides with PIFA, involving an electrophilic dearomative cascade cyclization.
![Graphical abstract: PIFA-mediated selenylative spirocyclization of indolyl ynones: facile access to selenated spiro[cyclopentenone-1,3′-indoles]](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D2RA05387J&imageInfo.ImageIdentifier.Year=2022)
RSC Adv., 2022,12, 28800-28803
https://doi.org/10.1039/D2RA05387J
A gold(I)-catalysed approach towards harmalidine an elusive alkaloid from Peganum harmala
Upon gold catalysis, the 2,3-dihydropyrrolo[1,2-a]indole motif, encountered in few but interesting bioactive natural products, was efficiently obtained from N-aryl 2-alkynylazetidine derivatives.
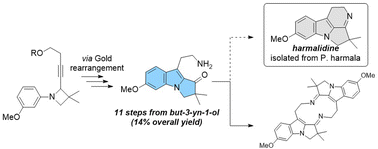
RSC Adv., 2022,12, 26966-26974
https://doi.org/10.1039/D2RA05685B
Metal-free synthesis of C2-quaternary indolinones by (NH4)2S2O8 mediated oxidative dearomatization of indoles
An efficient metal-free, (NH4)2S2O8 mediated oxidative dearomatization of indoles for construction of C2-quaternary indolinones was disclosed which provides an approach for generation of all-carbon quaternary centers at the C2 position of indoles.

RSC Adv., 2022,12, 21022-21025
https://doi.org/10.1039/D2RA04191J
Site-specific role of bifunctional graphitic carbon nitride catalyst for the sustainable synthesis of 3,3-spirocyclic oxindoles in aqueous media
Functionalized graphitic carbon nitride (Sg-C3N4) has been manufactured and used as a reusable catalyst for the one-pot production of various spiro-pyrano chromenes and spiro indole-3,1′-naphthalene tetracyclic systems in aqueous media.

RSC Adv., 2021,11, 28452-28465
https://doi.org/10.1039/D1RA03881H
PhI(OAc)2-mediated intramolecular oxidative C–N coupling and detosylative aromatization: an access to indolo[2,3-b]quinolines
Under mild conditions, PIDA mediated oxidant C–N coupling to afford indolo[2,3-b]quinoline derivatives has been developed with a decent substrate scope.
![Graphical abstract: PhI(OAc)2-mediated intramolecular oxidative C–N coupling and detosylative aromatization: an access to indolo[2,3-b]quinolines](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D1RA01894A&imageInfo.ImageIdentifier.Year=2021)
RSC Adv., 2021,11, 17206-17211
https://doi.org/10.1039/D1RA01894A
TCCA-mediated oxidative rearrangement of tetrahydro-β-carbolines: facile access to spirooxindoles and the total synthesis of (±)-coerulescine and (±)-horsfiline
Three-reactive centered reagent (TCCA) mediated construction of spirooxindoles through an oxidative rearrangement of various N-protected tetrahydro-β-carbolines and total synthesis of natural alkaloids (±)-coerulescine and (±)-horsfiline.

RSC Adv., 2021,11, 16537-16546
https://doi.org/10.1039/D1RA02381K
Dual C–H activation: Rh(III)-catalyzed cascade π-extended annulation of 2-arylindole with benzoquinone
A Rh-catalyzed cascade annulation of N–H free 2-arylindole with benzoquinone via dual C–H activation strategy was reported.

RSC Adv., 2021,11, 13030-13033
https://doi.org/10.1039/D1RA01779A
Rhodium(III) catalyzed olefination and deuteration of tetrahydrocarbazole
The rhodium-catalyzed olefination and deuteration of tetrahydrocarbazoles in water with the aid of an N,N-dimethylcarbamoyl-protected group is presented.

RSC Adv., 2021,11, 8356-8361
https://doi.org/10.1039/D1RA00236H
Palladium catalyzed reductive Heck coupling and its application in total synthesis of (−)-17-nor-excelsinidine
17-nor-Excelsinidine was constructed via oxidative coupling in excellent yield and high regioselectivity under NBS/pyridine from the enolate of geissoschizine.

RSC Adv., 2021,11, 7570-7574
https://doi.org/10.1039/D1RA00015B
Bifunctional thiosquaramide catalyzed asymmetric reduction of dihydro-β-carbolines and enantioselective synthesis of (−)-coerulescine and (−)-horsfiline by oxidative rearrangement
A simple and efficient asymmetric synthesis of THBCs through a chiral thiosquaramide 11b catalyzed imine reduction of dihydro-β-carbolines (17a−f) and syntheses of (−)-coerulescine and (–)-horsfiline via enantioselective oxidative rearrangement.
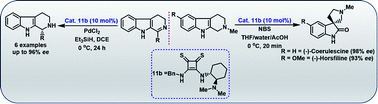
RSC Adv., 2020,10, 38672-38677
https://doi.org/10.1039/D0RA07705D
Synthesis of 1-(β-coumarinyl)-1-(β-indolyl)trifluoroethanols through regioselective Friedel–Crafts alkylation of indoles with β-(trifluoroacetyl)coumarins catalyzed by Sc(OTf)3
A Friedel–Crafts alkylation of indoles with β-(trifluoroacetyl)coumarins catalyzed by Sc(OTf)3 to afford 1-(β-coumarinyl)-1-(β-indolyl)trifluoroethanols in a short time and high yield was developed.

RSC Adv., 2020,10, 13929-13935
https://doi.org/10.1039/D0RA01237H
Access to N-unprotected 2-amide-substituted indoles from Ugi adducts via palladium-catalyzed intramolecular cyclization of o-iodoanilines bearing furan rings
A variety of N-unprotected 2-amide-substituted indoles were synthesized from readily available furfural-based Ugi adducts in moderate to good yields via palladium-catalyzed intramolecular cyclization of o-iodoanilines bearing furan rings.

RSC Adv., 2020,10, 11750-11754
https://doi.org/10.1039/D0RA01830A
Novel base-initiated cascade reactions of hemiindigos to produce dipolar γ-carbolines and indole-fused pentacycles
Based on hemiindigos we developed novel reactions for producing γ-carbolines and their precursors that appeared to be active against MTB.
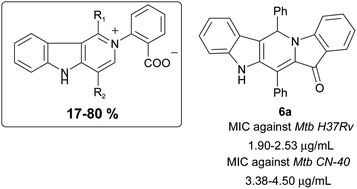
RSC Adv., 2019,9, 41402-41408
https://doi.org/10.1039/C9RA07807J
Microwave-assisted synthesis of 7-azaindoles via iron-catalyzed cyclization of an o-haloaromatic amine with terminal alkynes
An efficient and practical procedure was developed to prepare 7-azaindole, starting from an o-haloaromatic amine and corresponding terminal alkynes under microwave irradiation and the scope was demonstrated with a number of examples.

RSC Adv., 2019,9, 39684-39688
https://doi.org/10.1039/C9RA08742G
Access to indolines from primary phenylethylamines by an unexpected palladium-catalyzed C–H functionalization process
A new method for the preparation of 2,2-disubstituted indolines from 2-phenylethylamines was developed under Pd catalysis and PhI(OAc)2 as oxidant.

RSC Adv., 2019,9, 27176-27182
https://doi.org/10.1039/C9RA05670J
Synthesis of C2-tetrasubstituted indolin-3-ones via Cu-catalyzed oxidative dimerization of 2-aryl indoles and cross-addition with indoles
A general protocol for the synthesis of 2,2-disubstituted indolin-3-ones has been developed through self-dimerization of 2-aryl indoles and cross-addition with indoles under mild Cu-catalyzed oxidative conditions with good to high yields.
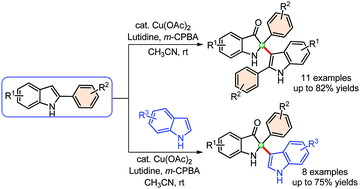
RSC Adv., 2019,9, 24050-24056
https://doi.org/10.1039/C9RA04741G
A facile approach to 2-alkoxyindolin-3-one and its application to the synthesis of N-benzyl matemone
A short-step synthesis of N-benzyl matemone has been successfully carried out using a newly discovered 2-ethoxycarbonylindolin-3-one synthesis.

RSC Adv., 2019,9, 17341-17346
https://doi.org/10.1039/C9RA02204J
Synthesis of spiroimidazopyridineoxindole, spiropyridopyrimidineoxindole and spiropyridodiazepineoxindole derivatives based on heterocyclic ketene aminals via a four-component reaction
A simple and efficient approach to the synthesis of three new classes of fused spiropyridineoxindoles using heterocyclic ketene aminals.

RSC Adv., 2019,9, 16384-16389
https://doi.org/10.1039/C8RA10379H
A nitroalkane-based approach to one-pot three-component synthesis of isocryptolepine and its analogs with potent anti-cancer activities
An improved one-pot three-component synthesis involving electrophilically activated nitroalkanes allowed for efficient preparation of indoloquinolines with potent anticancer activities.

RSC Adv., 2018,8, 36980-36986
https://doi.org/10.1039/C8RA08155G
Diastereoselective construction of 4-indole substituted chromans bearing a ketal motif through a three-component Friedel–Crafts alkylation/ketalization sequence
The first TfOH-catalyzed three-component Friedel–Crafts alkylation/ketalization sequence of indoles, alcohols and ortho-hydroxychalcones was developed to afford a wide range of 4-indole substituted chromans bearing a ketal motif in 77–99% yields.
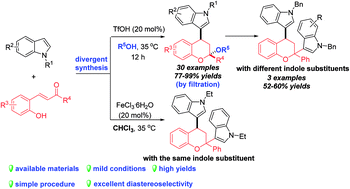
RSC Adv., 2018,8, 15641-15651
https://doi.org/10.1039/C8RA02481B
N,N-Dimethylformamide-stabilized palladium nanoclusters as a catalyst for Larock indole synthesis
Pd NCs serve recyclable and highly active catalyst to indole synthesis.

RSC Adv., 2018,8, 11324-11329
https://doi.org/10.1039/C8RA01410H
Synthesis of 3-arylindole derivatives from nitroalkane precursors
3-Arylindole derivatives were synthesized by Cu(I) catalysed intramolecular Ullmann coupling of 2-bromoarylaminoalkanes.

RSC Adv., 2016,6, 96049-96056
https://doi.org/10.1039/C6RA21144E
Metal-free 1,3-dipolar cycloaddition approach towards the regioselective synthesis of β-carboline and isoxazole based molecular hybrids
Nature has nourished β-carboline and isoxazole derivatives as privileged scaffolds and consequently they are ubiquitously found in alkaloids isolated from various sources.
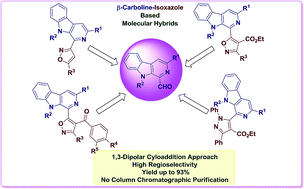
RSC Adv., 2016,6, 88066-88076
https://doi.org/10.1039/C6RA15875G
A novel self-terminated Prins strategy for the synthesis of tetrahydropyran-4-one derivatives and their behaviour in Fisher indole synthesis
A novel self-terminated Prins strategy has been developed for the synthesis of 2-substituted tetrahydropyran-4-one derivatives through a condensation of 3-(phenylthio)but-3-en-1-ol with carbonyl compounds in the presence of 5 mol% of Sc(OTf)3 under mild conditions.

RSC Adv., 2016,6, 75133-75137
https://doi.org/10.1039/C6RA11218H
Synthesis of spiro isoindolinone-indolines and 1,2-disubstituted indoles from 2-iodobenzamide derivatives
Copper catalyzed C-terminal and N-terminal attack of 2-alkynylanilines to 2-iodobenzamides afforded isoindolinones and 1,2-disubstituted indoles, respectively.
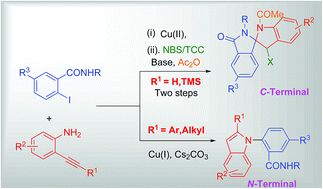
RSC Adv., 2016,6, 74845-74858
https://doi.org/10.1039/C6RA15002K
Highly selective intramolecular addition of C–N and S–N bonds to alkynes catalyzed by palladium: a practical access to two distinct functional indoles
Palladium-catalyzed highly selective intramolecular addition of C–N and S–N bonds to alkynes has been achieved, and two distinct kinds of functional indoles were synthesized rapidly from the same set of substrates in a catalyst-controlled manner.

RSC Adv., 2016,6, 70682-70690
https://doi.org/10.1039/C6RA15945A
Collective formal synthesis of (±)-rhynchophylline and homologues
A collective formal synthesis approach to six bioactive oxindole alkaloids, including (±)-rhynchophylline and its five homologues, is completed in a highly efficient way.
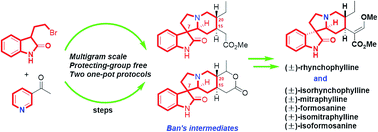
RSC Adv., 2016,6, 63131-63135
https://doi.org/10.1039/C6RA12766E
L-Isoleucine derived bifunctional phosphine catalyses asymmetric [3 + 2]-annulation of allenyl-esters and -ketones with ketimines
L-Isoleucine derived bifunctional N-acylaminophosphine catalyzed a [3 + 2]-annulation reaction between allenyl carbonyl compounds and isatinimines to afford a facile and asymmetric access to 3,2′-dihydropyrrolyl spirooxindoles.
![Graphical abstract: l-Isoleucine derived bifunctional phosphine catalyses asymmetric [3 + 2]-annulation of allenyl-esters and -ketones with ketimines](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6RA12387B&imageInfo.ImageIdentifier.Year=2016)
RSC Adv., 2016,6, 56537-56543
https://doi.org/10.1039/C6RA12387B
β-Carboline-directed decarboxylative acylation of ortho-C(sp2)–H of the aryl ring of aryl(β-carbolin-1-yl)methanones with α-ketoacids under palladium catalysis
A Pd-catalysed β-carboline assisted decarboxylative acylation of ortho-C(sp2)–H of aryl ring of aryl(β-carbolin-1-yl)methanones using α-oxocarboxylic acid as the acyl ion source to form (2-aroylaryl)(β-carbolin-2-yl)methanones is demonstrated.

RSC Adv., 2016,6, 50658-50665
https://doi.org/10.1039/C6RA11811A
Rhodium-catalyzed cycloaddition of carbonyl ylides for the synthesis of spiro[furo[2,3-a]xanthene-2,3′-indolin]-2′-one scaffolds
Rh(II)-catalyzed cycloaddition of 3-diazooxindoles with 2-methyl-2-(4-methylpent-3-en-1-yl)-2H-chromene-3-carbaldehydes has been developed to produce tetrahydro-3H,4H-spiro[furo[2,3-a]xanthene-2,3′-indolin]-2′-ones in good yields.
![Graphical abstract: Rhodium-catalyzed cycloaddition of carbonyl ylides for the synthesis of spiro[furo[2,3-a]xanthene-2,3′-indolin]-2′-one scaffolds](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6RA05661J&imageInfo.ImageIdentifier.Year=2016)
RSC Adv., 2016,6, 50497-50499
https://doi.org/10.1039/C6RA05661J
A concise approach to indoles via oxidative C–H amination of 2-alkenylanilines using dioxygen as the sole oxidant
An operationally simple and environmental friendly approach to indole derivatives from N-Ts-2-alkenylanilines has been achieved, which involves an oxidative intramolecular C–H amination by using molecular oxygen as sole oxidant.

RSC Adv., 2016,6, 35764-35770
https://doi.org/10.1039/C6RA03378D
A cascade reaction for the new and direct synthesis of indolofuroquinoxalines
A cascade reaction has been developed for the direct and one-pot synthesis of 7H-indolo[3′,2′:4,5]furo[2,3-b]quinoxaline derivatives.

RSC Adv., 2016,6, 23489-23497
https://doi.org/10.1039/C6RA03556F
Diversity oriented synthesis of β-carbolinone and indolo-pyrazinone analogues based on an Ugi four component reaction and subsequent cyclisation of the resulting indole intermediate
One pot two step synthesis of β-carbolinone and indolo-pyrazinone analogues via acid mediated cyclisation of Ugi intermediate has been developed with a wide substrate scope.
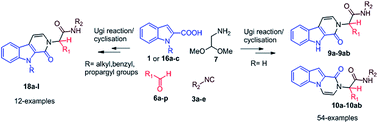
RSC Adv., 2016,6, 21165-21186
https://doi.org/10.1039/C5RA27090A
About this collection
RSC Advances is delighted to present this themed collection, edited jointly by Dr. Sarbani Pal (MNR Degree and PG College, India) and Associate Editor Dr. Manojit Pal (Dr Reddy’s Institute of Life Sciences, India).
Indoles are attractive targets in organic synthesis because of not only their widespread existences in nature especially in alkaloids but also their importance as privileged structures in Medicinal / Pharmaceutical Chemistry and Drug Discovery. It is not surprising that the indole framework is a commonly found N-heteroarene moiety in many bioactive agents and drugs. Additionally, many indoles served as key precursors to a range of valuable compounds that find applications in various areas of science. Thus enormous efforts have been devoted for the development of elegant synthetic routes to various indole derivatives or indole based complex structures. This current web collection is mainly a compilation of relevant important and interesting research papers already published in RSC Advances during last 7 years. The major focus of this compilation was on selection of the elegant synthetic methods including single or multi-step approaches, multi-component reactions, transition or other metal catalysed methods, cascade reactions, environmentally friendly approaches etc reported for indole derivatives. The reports on simple or mere derivatization / functionalization of indole ring are generally excluded. Some selected papers reporting synthesis as well as biological activities of indole derivatives are also included.
RSC Advances welcome research papers on new and latest developments in the area of indole synthesis for further inclusion in the web collection. If you are interested in submitting to this collection, please contact the Editorial Office at advances-rsc.org