Scandium triflate-catalyzed selective ring opening and rearrangement reaction of spiro-epoxyoxindole and carbonyl compounds†
Abstract
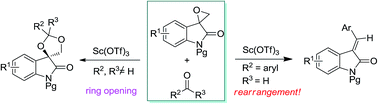
Scandium triflate-catalyzed reactions between spiro-epoxyoxindole and carbonyl compounds have been disclosed. The reaction with ketones can be used to synthesize spiro[[1,3]dioxolane-4,3′-indolin] species. Furthermore, an unprecedented rearrangement takes place to yield alkylidene oxindole when aromatic aldehydes are used as carbonyl components.
- This article is part of the themed collection: Elegant Synthetic Routes to Indole Derivatives

 Please wait while we load your content...
Please wait while we load your content...