Bifunctional thiosquaramide catalyzed asymmetric reduction of dihydro-β-carbolines and enantioselective synthesis of (−)-coerulescine and (−)-horsfiline by oxidative rearrangement†
Abstract
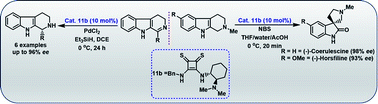
Tetrahydro-β-carboline (THBC) is a tricyclic ring system that can be found in a large number of bioactive alkaloids. Herein, we report a simple and efficient method for the synthesis of enantiopure THBCs through a chiral thiosquaramide (11b) catalyzed imine reduction of dihydro-β-carbolines (17a–f). The in situ generated Pd–H employed as hydride source in the reaction of differently substituted chiral THBCs (18a–f) afforded high selectivities (R isomers, up to 96% ee) and good isolated yields (up to 88%). Moreover, the chiral thiosquaramide used also afforded exceptional catalyst activity in the syntheses of (−)-coerulescine (5) and (−)-horsfiline (6) with excellent enantioselectivities up to 98% and 93% ee, respectively, via an enantioselective oxidative rearrangement approach.
- This article is part of the themed collection: Elegant Synthetic Routes to Indole Derivatives


 Please wait while we load your content...
Please wait while we load your content...