Themed collection Reaction mechanisms in catalysis

Concluding remarks: Reaction mechanisms in catalysis: perspectives and prospects
A combination of powerful computational and experimental techniques is revealing the mechanistic details of key catalytic reactions.

Faraday Discuss., 2021,229, 502-513
https://doi.org/10.1039/D1FD00027F
Spiers Memorial Lecture: Understanding reaction mechanisms in heterogeneously catalysed reactions
Heterogeneous catalysis lies at the heart of the chemical and fuel manufacturing industries and hence is a cornerstone of many economies.
Faraday Discuss., 2021,229, 9-34
https://doi.org/10.1039/D1FD00023C
Surface stability of perovskite oxides under OER operating conditions: a first principles approach
Understanding the surface stability of perovskite oxides under OER operating conditions is crucial for the atomistic design of electrocatalysts for electrochemical water-splitting.
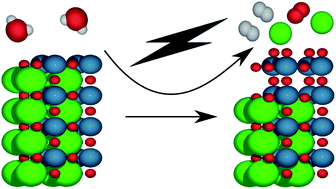
Faraday Discuss., 2021,229, 75-88
https://doi.org/10.1039/C9FD00146H
Reaction mechanism of low-temperature catalysis by surface protonics in an electric field
We investigate the mechanism behind the high catalytic activities achieved when combining heterogeneous catalysts and direct current electric fields even under mild conditions (<500 K) with relatively low electrical energy consumption.
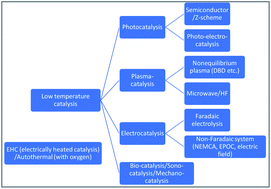
Faraday Discuss., 2021,229, 341-358
https://doi.org/10.1039/C9FD00129H
Multi-nuclear, high-pressure, operando FlowNMR spectroscopic study of Rh/PPh3 – catalysed hydroformylation of 1-hexene
The hydroformylation of 1-hexene with 12 bar of 1 : 1 H2/CO in the presence of the catalytic system [Rh(acac)(CO)2]/PPh3 was successfully studied by real-time multinuclear high-resolution FlowNMR spectroscopy at 50 °C.
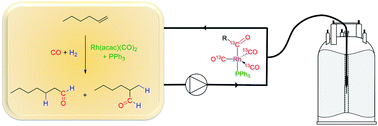
Faraday Discuss., 2021,229, 422-442
https://doi.org/10.1039/C9FD00145J
Capturing spatially resolved kinetic data and coking of Ga–Pt supported catalytically active liquid metal solutions during propane dehydrogenation in situ
Spatially resolved kinetic data of gallium–platinum SCALMS was captured while elucidating the effect of carrier material on coke formation.
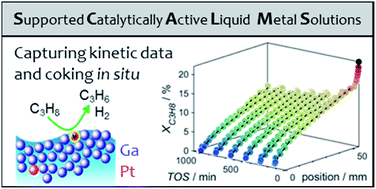
Faraday Discuss., 2021,229, 359-377
https://doi.org/10.1039/D0FD00010H
CO2 reduction to acetic acid on the greigite Fe3S4{111} surface
The greigite Fe3S4{111} surface catalyses the CO2 conversion into acetic acid (CH3–COOH) via a glyoxylic acid (CHO–COOH) intermediate.
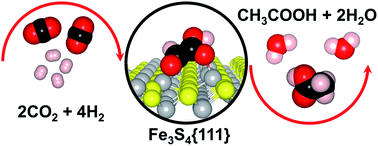
Faraday Discuss., 2021,229, 35-49
https://doi.org/10.1039/C9FD00141G
A combined periodic DFT and QM/MM approach to understand the radical mechanism of the catalytic production of methanol from glycerol
Hydroxymethyl radicals deprotonate over MgO(100) and can disproportionate to methanol and formaldehyde; DFT calculated pathway matched to experimental product analysis.
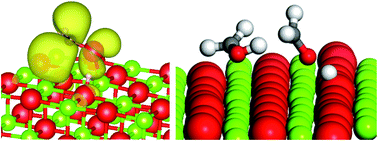
Faraday Discuss., 2021,229, 108-130
https://doi.org/10.1039/D0FD00005A
The interaction of CO with a copper(II) chloride oxy-chlorination catalyst
The interaction of CO with an attapulgite-supported, KCl modified CuCl2 catalyst is investigated via a series of micro-reactor measurements.

Faraday Discuss., 2021,229, 318-340
https://doi.org/10.1039/D0FD00014K
Single catalyst particle diagnostics in a microreactor for performing multiphase hydrogenation reactions
The single particle hydrogenation of methylene blue over a Pd/SiO2 catalyst was monitored in a droplet-microreactor, using red/green/blue optical microscopy.
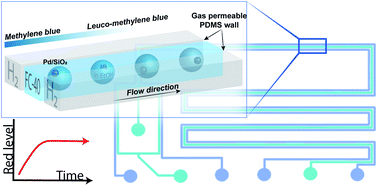
Faraday Discuss., 2021,229, 267-280
https://doi.org/10.1039/D0FD00006J
Hydrogenation of ethylene over palladium: evolution of the catalyst structure by operando synchrotron-based techniques
We study a carbon-supported palladium-based catalyst during the hydrogenation of ethylene to ethane in a wide range of partial pressures of ethylene and hydrogen.
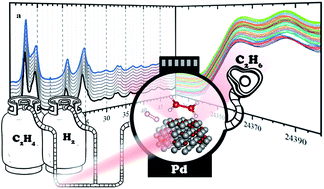
Faraday Discuss., 2021,229, 197-207
https://doi.org/10.1039/C9FD00139E
Visualization of catalyst dynamics and development of a practical procedure to study complex “cocktail”-type catalytic systems
We propose a simple method for in situ capturing of nanoparticles with carbon-coated grids directly from reaction mixtures.

Faraday Discuss., 2021,229, 458-474
https://doi.org/10.1039/C9FD00125E
The effect of H2 : N2 ratio on the NH3 synthesis rate and on process economics over the Co3Mo3N catalyst
Ammonia synthesis rates are independent of H2 : N2 stoichiometry for H2 : N2 > 0.5 over the Co3Mo3N catalyst. Only strongly adsorbed hydrogen is involved in the reaction. Its coverage is independent of hydrogen pressure for H2 : N2 > 0.5.
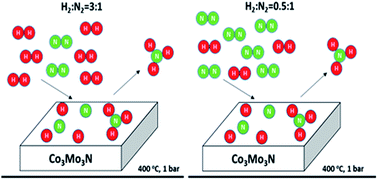
Faraday Discuss., 2021,229, 475-488
https://doi.org/10.1039/C9FD00136K
Mechanistic in situ investigation of heterogeneous hydrogenation over Rh/TiO2 catalysts: selectivity, pairwise route and catalyst nature
We report a catalyst with the highest selectivity toward pairwise hydrogen addition of 7% among supported metal catalysts, found as a result of variation of Rh/TiO2 catalyst preparation procedures.
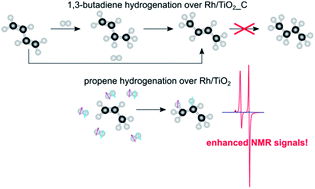
Faraday Discuss., 2021,229, 161-175
https://doi.org/10.1039/C9FD00138G
Effect of thermal treatment on the stability of Na–Mn–W/SiO2 catalyst for the oxidative coupling of methane
The effect of different calcination protocols on the performance of a Na–Mn–W/SiO2 catalyst is studied with laboratory and synchrotron X-ray based characterisation techniques.
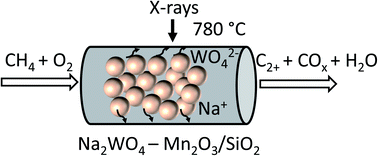
Faraday Discuss., 2021,229, 176-196
https://doi.org/10.1039/C9FD00142E
Combination of theoretical and in situ experimental investigations of the role of lithium dopant in manganese nitride: a two-stage reagent for ammonia synthesis
This article presents an investigation of the role of dopant in manganese nitride, a two-stage reagent for ammonia synthesis, by a combination of theoretical and in situ experimental investigations.

Faraday Discuss., 2021,229, 281-296
https://doi.org/10.1039/C9FD00131J
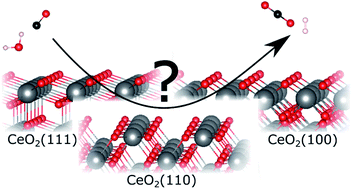
Insight into the mechanism of the water–gas shift reaction over Au/CeO2 catalysts using combined operando spectroscopies
The mechanism of the low-temperature water–gas shift (LT-WGS) reaction over Au/CeO2 catalysts with different ceria terminations, i.e., (111), (110), and (100) facets, was investigated.
Faraday Discuss., 2021,229, 232-250
https://doi.org/10.1039/C9FD00133F
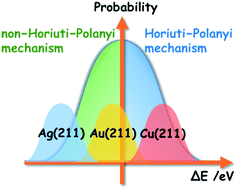
Addressing the uncertainty of DFT-determined hydrogenation mechanisms over coinage metal surfaces
Using model reactions and employing a well-trained Bayesian error estimation functional with van der Waals correlation, we estimate the error of DFT calculation results statistically, and therefore predict the reliability of the hydrogenation mechanisms identified.
Faraday Discuss., 2021,229, 50-61
https://doi.org/10.1039/C9FD00122K
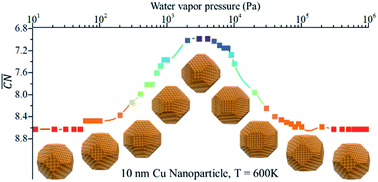
Structure reconstruction of metal/alloy in reaction conditions: a volcano curve?
The gas-induced structure reconstruction of metal/alloy evolves like a volcano curve in response to temperature or gas pressure.
Faraday Discuss., 2021,229, 62-74
https://doi.org/10.1039/C9FD00128J
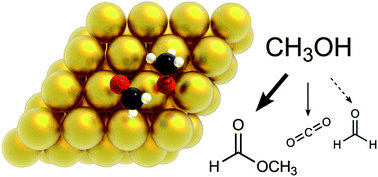
The role of oxygenated species in the catalytic self-coupling of MeOH on O pre-covered Au(111)
Density functional theory and kinetic Monte Carlo simulation elucidate the complex pathways of methanol oxidation, as well as the role of different oxygenates in this chemistry, which is important in the valorisation of biomass.
Faraday Discuss., 2021,229, 251-266
https://doi.org/10.1039/C9FD00134D
Supported FexNiy catalysts for the co-activation of CO2 and small alkanes
The required tandem functionality between FeNi species and a reducible oxidic carrier in the CO2-ODH is demonstrated.
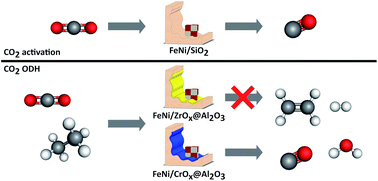
Faraday Discuss., 2021,229, 208-231
https://doi.org/10.1039/C9FD00130A
A multiscale modelling approach to elucidate the mechanism of the oxygen evolution reaction at the hematite–water interface
A novel multiscale model to elucidate the mechanism of the oxygen evolution reaction at the hematite–water interface.
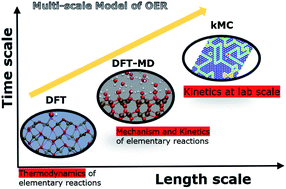
Faraday Discuss., 2021,229, 89-107
https://doi.org/10.1039/C9FD00140A
Elucidating the role of H2O in promoting the formation of methacrylic acid during the oxidation of methacrolein over heteropolyacid compounds
The involvement of water in the selective oxidation of MAL to MAA over a pure Keggin-type H3PMoO12O40 catalyst was investigated using an in situ DRIFTS reactor coupled with a mass spectrometer for the first time to elucidate the reaction pathway associated with water.
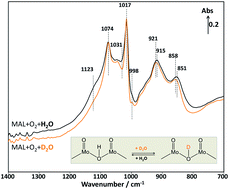
Faraday Discuss., 2021,229, 443-457
https://doi.org/10.1039/C9FD00135B
Hydrogenation of substituted nitroaromatics on non-noble metal catalysts: mechanistic insights to improve selectivity
An alternative reaction mechanism combined with preferential perpendicular adsorption make non-noble metals chemoselective catalysts for nitroarene hydrogenation.
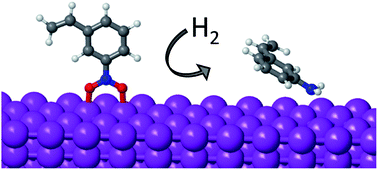
Faraday Discuss., 2021,229, 297-317
https://doi.org/10.1039/C9FD00126C
Advanced approaches: general discussion
Faraday Discuss., 2021,229, 378-421
https://doi.org/10.1039/D1FD90032C
Dynamics: general discussion
Faraday Discuss., 2021,229, 489-501
https://doi.org/10.1039/D1FD90031E
Theory: general discussion
Faraday Discuss., 2021,229, 131-160
https://doi.org/10.1039/D1FD90030G
About this collection
We are delighted to share with you a selection of the papers associated with a Faraday Discussion on Reaction mechanisms in catalysis. More information about the related event may be found here: http://rsc.li/catalysis-fd2020. Additional articles will be added to the collection as they are published. The final versions of all the articles presented and a record of the live discussions will be published after the event.
Heterogeneous catalysis is a core area of contemporary physical chemistry posing major fundamental and conceptual challenges. Catalysis lies at the heart of the chemical industry - an immensely successful and important part of the overall UK economy, and catalysis plays a crucial part in the production of 80% of all manufactured goods. Catalysis is a major theme in chemical sciences and engineering that underlies much of the key research and teaching in these subjects.
The reaction mechanisms of many commercial processes although successfully operated, are still a matter of debate and controversy, e.g. methanol synthesis and Fischer Tropsch catalysts. Hence there is now the opportunity to focus a Faraday Discussion concerning key aspects of reaction mechanism studies and how this can drive rational design of catalysts.