Reaction mechanism of low-temperature catalysis by surface protonics in an electric field
Abstract
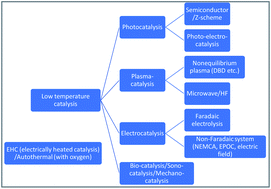
The process of combining heterogeneous catalysts and direct current (DC) electric fields can achieve high catalytic activities, even under mild conditions (<500 K) with relatively low electrical energy consumption. Hydrogen production by steam reforming of methane, aromatics and alcohol, dehydrogenation of methylcyclohexane, dry reforming of methane, and ammonia synthesis are known to proceed at low temperatures in an electric field. In situ/operando analyses are conducted using IR, Raman, X-ray absorption fine structure, electrochemical impedance spectroscopy, and isotopic kinetic analyses to elucidate the reaction mechanism for these reactions at low temperatures. The results show that surface proton hopping by a DC electric field, called surface protonics, is important for these reactions at low temperatures because of the higher surface adsorbate concentrations at lower temperatures.
- This article is part of the themed collection: Reaction mechanisms in catalysis


 Please wait while we load your content...
Please wait while we load your content...