Elucidating the role of H2O in promoting the formation of methacrylic acid during the oxidation of methacrolein over heteropolyacid compounds†
Abstract
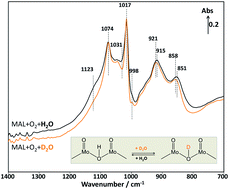
The involvement of water in the selective oxidation of MAL to MAA over a pure Keggin-type H3PMoO12O40 catalyst was investigated using an in situ DRIFTS reactor coupled with a mass spectrometer for the first time to elucidate the reaction pathway associated with water. Comparing the spectra and activity data using D2O instead of H2O during transient switching experiments has allowed us to evaluate the possible active sites where D2O is activated. It has been found that, during the cycling switches of D2O in and out of the MAL + O2 gas feed at 320 °C, the formation of MAA–OD product is increased and decreased when D2O is added and removed, respectively. This suggests that the deuterium from D2O is involved in the production of gas phase MAA–OD. In addition, the in situ DRIFTS-MS results obtained from the isotopic switches between D2O and H2O reveal changes in the characteristic infrared bands of the Keggin unit between 1200 and 600 cm−1. It is found that the isotopic exchange possibly occurs on the bridging oxygen of Mo–O–Mo unit, where water is activated for the formation of MAA. Based on the in situ DRIFTS-MS analysis from the transient switching experiments, the reaction mechanism associated with the effect of water on the selective oxidation of MAL to MAA over Keggin-type H3PMoO12O40 catalyst is proposed.
- This article is part of the themed collection: Reaction mechanisms in catalysis


 Please wait while we load your content...
Please wait while we load your content...