Themed collection Foldamer Chemistry

Introduction to the themed issue on Foldamer Chemistry
This themed issue of NJC features a collection of papers highlighting the latest developments in foldamer chemistry.

New J. Chem., 2015,39, 3188-3189
https://doi.org/10.1039/C5NJ90019K
Single and multiple peptide γ-turns: literature survey and recent progress
Published data on peptide isolated and repetitive γ-turns are reviewed. Advancements in our laboratories on these 3D-structures are also presented.
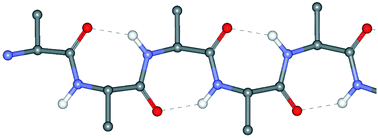
New J. Chem., 2015,39, 3208-3216
https://doi.org/10.1039/C4NJ01564A
Model foldamers: applications and structures of stable macrocyclic peptides identified using in vitro selection
A survey of crystal- and solution-structure information for macrocyclic peptides, illustrating common folding patterns and target binding effects.
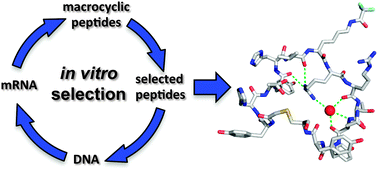
New J. Chem., 2015,39, 3197-3207
https://doi.org/10.1039/C4NJ01633E
Conformational properties of aromatic multi-layered and helical oligoureas and oligoguanidines
Cis conformational properties of N,N′-dimethylurea and guanidine can be applied to construct foldamers with aromatic multilayers and dynamic helical conformations.
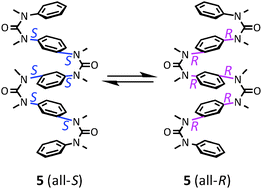
New J. Chem., 2015,39, 3190-3196
https://doi.org/10.1039/C4NJ01885K
Photo-crosslinking of a self-assembled coumarin-dipeptide hydrogel
The photo-crosslinking of a coumarin-functionalized dipeptide hydrogel enhances the stability of the self-assembled nanofibers that comprise the hydrogel.
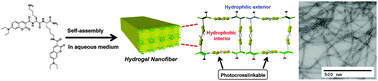
New J. Chem., 2015,39, 3225-3228
https://doi.org/10.1039/C5NJ00038F
cis-2-Aminocyclohex-4-enecarboxylic acid as a new building block of helical foldamers
cis-2-Aminocyclohex-4-enecarboxylic acid can promote the α/β-peptide 11/9-helix in solution and in the crystal state.
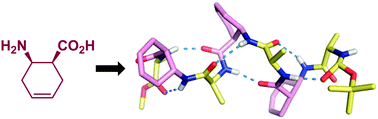
New J. Chem., 2015,39, 3221-3224
https://doi.org/10.1039/C4NJ02056A
Aromatic oligoamides with increased backbone flexibility: improved synthetic efficiencies, solvent-dependent folding and cooperative conformational transitions
A 15-residue aromatic oligoamide with a backbone of increased flexibility exhibits solvent- and temperature-dependent folding and highly cooperative conformational transition.
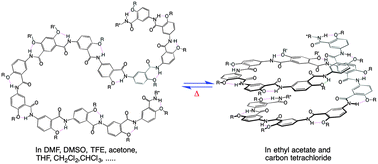
New J. Chem., 2015,39, 3217-3220
https://doi.org/10.1039/C4NJ01820F
Helical arylamide foldamers: structure prediction by molecular dynamics simulations
Snapshots from molecular dynamics simulations showcase how substituent positions and linkage types affect the secondary structure properties of fluorobenzene based helical arylamides.
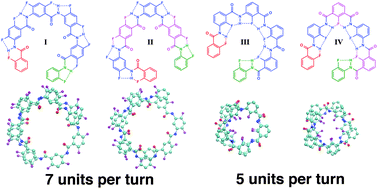
New J. Chem., 2015,39, 3229-3240
https://doi.org/10.1039/C4NJ01925C
The role of N-terminal proline in stabilizing the Ant–Pro zipper motif
This paper deals with the role of N-terminal proline in stabilizing the Ant–Pro zipper structure by the co-operative contribution of competing forces viz. hydrogen bonding, aromatic stacking and backbone chirality.
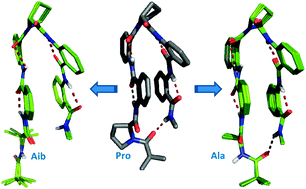
New J. Chem., 2015,39, 3327-3332
https://doi.org/10.1039/C4NJ02151G
Structural characterization of folded and extended conformations in peptides containing γ amino acids with proteinogenic side chains: crystal structures of γn, (αγ)n and γγδγ sequences
γn amino acid residues can be incorporated into structures in γn and hybrid sequences containing folded and extended α and δ residues.
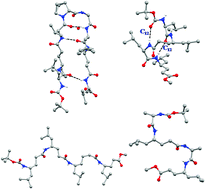
New J. Chem., 2015,39, 3319-3326
https://doi.org/10.1039/C5NJ00132C
Synthesis of enantiomerically pure δ-benzylproline derivatives
The methodology allows for the preparation of enantiopure δ-substituted L-proline analogues bearing the side chain of proteinogenic residues.
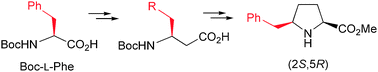
New J. Chem., 2015,39, 3310-3318
https://doi.org/10.1039/C4NJ01894J
Synthesis and conformational studies of α/β2,3-peptides derived from alternating β2,3-amino acids and L-Ala repeats
A new class of α/β2,3-peptides were synthesized from C-linked carbo-β2,3-amino acids (β2,3-Caas) and investigated to understand the impact of the side chains (allyl/propargyl) at the Cα-position on their conformations.

New J. Chem., 2015,39, 3295-3309
https://doi.org/10.1039/C4NJ02031F
Participation of non-aminoisobutyric acid (Aib) residues in the 310 helical conformation of Aib-rich foldamers: a solid state study
310 helical conformations that extend over 21 Å result when selected non-Aib terminal and central residues are incorporated into Aib-rich foldamers.
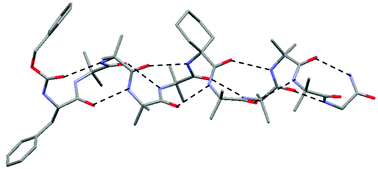
New J. Chem., 2015,39, 3288-3294
https://doi.org/10.1039/C4NJ01547A
Supramolecular self-assembly of 14-helical nanorods with tunable linear and dendritic hierarchical morphologies
Varying the solvent offers a simple way to control superstructure polymorphism of a tri-β3-peptide-based supramolecular system.
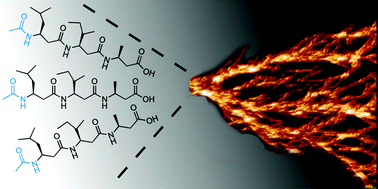
New J. Chem., 2015,39, 3280-3287
https://doi.org/10.1039/C4NJ01926A
Conformational preferences in the β-peptide oligomers of cis-2-amino-1-fluorocyclobutane-1-carboxylic acid
These oligomers adopt a regular zig-zag strand-like secondary structure which does not rely on intra-residue 6-ring hydrogen bonds for stability.
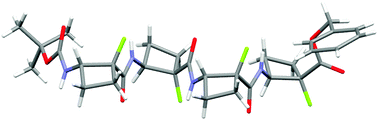
New J. Chem., 2015,39, 3270-3279
https://doi.org/10.1039/C4NJ01929F
Synthesis of a double-stranded spiroborate helicate bearing stilbene units and its photoresponsive behaviour
The sodium ion-triggered extension and contraction motions along with photo-induced cis–trans isomerisation of a photoresponsive spiroborate-based double-stranded helicate were investigated.
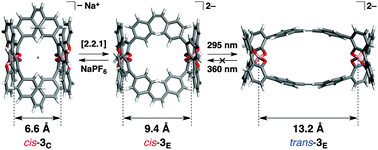
New J. Chem., 2015,39, 3259-3269
https://doi.org/10.1039/C4NJ01669F
Synthesis and conformational studies of a stable peptidomimetic β-hairpin based on a bifunctional diketopiperazine turn inducer
A new β-hairpin mimic foldamer based on the assembly of a reverse turn inducer, a peptidomimetic strand, and a tetrapeptide sequence was prepared, and its conformation in solution was assessed by NMR and computational investigations.
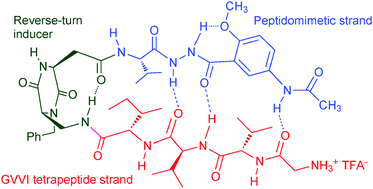
New J. Chem., 2015,39, 3250-3258
https://doi.org/10.1039/C4NJ01437E
Helix foldamers of γ-peptides based on 2-aminocyclopentylacetic acid
Oligo-γ-peptides based on 2-aminocyclopentylacetic acid (γAc5a) with a cyclopentyl constraint on the Cβ–Cγ bond and homochiral (1S,2S) configurations preferentially adopt the right-handed 14-helix foldamers in the gas phase and in solution.
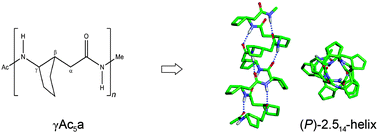
New J. Chem., 2015,39, 3241-3249
https://doi.org/10.1039/C4NJ01202J
About this collection
This themed issue of NJC features a collection of papers highlighting the latest developments in foldamer chemistry.