Helix foldamers of γ-peptides based on 2-aminocyclopentylacetic acid†
Abstract
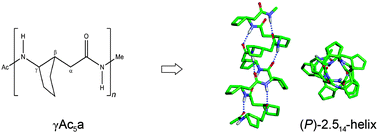
The conformational preferences of helix foldamers have been studied in oligo-γ-peptides composed of 2-aminocyclopentylacetic acid (γAc5a) with a cyclopentyl constraint on the Cβ–Cγ bond using density functional methods. Although the γAc5a (1) dipeptide with homochiral (1S,2S) configurations exhibits a strong preference for the right-handed (P) 9-membered helix foldamer in chloroform and water, oligopeptides composed of γAc5a (1) preferentially adopt (P)-2.514-helices due to the favored conformational energy produced as the peptide sequence becomes longer and as solvent polarity increases. The calculated mean backbone torsion angles and helical parameters for 14-helical foldamers composed of γAc5a (1) are consistent with those measured in the X-ray structures of penta- and hexapeptides composed of Cα-ethyl-γAc6a and 14-helical foldamers composed of γAc6a (1) with cyclohexyl constraints. However, the substitution of cyclopentane constraints in the backbone differentially changed the conformational preference and/or handedness for helix foldamers when compared with other oligo-γ-peptides with different cycloalkane positions or size constraints. The conformational preferences of the oligo-γAc5a peptides obtained here are expected to provide useful information for the structure-based design of biologically active γ-peptides with specific functions.
- This article is part of the themed collection: Foldamer Chemistry

 Please wait while we load your content...
Please wait while we load your content...