Synthesis of enantiomerically pure δ-benzylproline derivatives†
Abstract
The (2S,5R) stereoisomer of 5-benzylproline, i.e. the L-proline analogue that bears a δ-benzyl substituent cis to the carbonyl function, has been prepared in enantiomerically pure form and excellent global yield. The procedure involves the construction of the pyrrolidine ring through intramolecular cyclization and uses as starting material the enantiopure β-amino acid obtained by homologation of L-phenylalanine. The generation of an intermediate vinyl triflate with full regiochemical control followed by a stereoselective hydrogenation reaction allowed the isolation of the target δ-substituted L-proline analogue in optically pure form and 43% overall yield from the initial β-amino acid. The trans stereoisomer of (2R,5R) configuration is obtained as a minor product through a less stereoselective hydrogenation reaction.
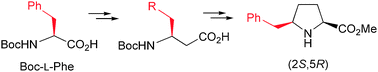
- This article is part of the themed collection: Foldamer Chemistry

 Please wait while we load your content...
Please wait while we load your content...