Conformational properties of aromatic multi-layered and helical oligoureas and oligoguanidines
Abstract
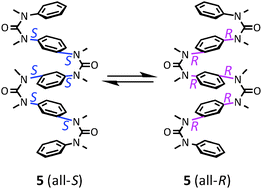
The use of N,N′-dialkylation of aromatic ureas and guanidines to achieve consecutive stereochemical switching from (trans,trans) to (cis,cis) conformations provides an elegant methodology for constructing ladder-like multi-layered aromatic oligoureas and oligoguanidines. Here, we highlight the structural properties and functions of these stable, flexible oligomers, which are candidate backbone structures for novel functional materials including electronic devices, chiral recognition, and bioactive substances.
- This article is part of the themed collection: Foldamer Chemistry

 Please wait while we load your content...
Please wait while we load your content...