Themed collection Asymmetric catalysis

Asymmetric one-pot reactions using heterogeneous chemical catalysis: recent steps towards sustainable processes
Asymmetric one-pot reactions applying heterogeneous chemical catalysts and unifying the benefits of these catalytic materials with the advantages of one-pot methods, are surveyed.

Catal. Sci. Technol., 2018,8, 389-422
https://doi.org/10.1039/C7CY01671A
The role of biocatalysis in the asymmetric synthesis of alkaloids – an update
This review article discusses developments in the chemo-enzymatic synthesis of alkaloids since 2013, showcasing how modern methods of organic synthesis and biocatalysis are combined to establish novel routes towards these important natural products.
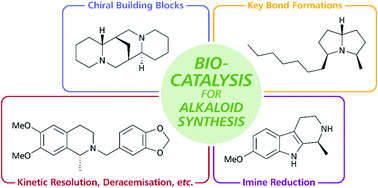
RSC Adv., 2021,11, 28223-28270
https://doi.org/10.1039/D1RA04181A
Asymmetric catalysis in direct nitromethane-free Henry reactions
This review summarizes the current state and applications of catalytic Henry reactions involving complex nitroalkanes coupling with various carbonyl compounds to generate chiral β-nitro alcohol scaffolds with four adjacent stereogenic centers.
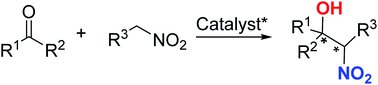
RSC Adv., 2020,10, 2313-2326
https://doi.org/10.1039/C9RA10263A
Application of asymmetric Sharpless aminohydroxylation in total synthesis of natural products and some synthetic complex bio-active molecules
This work shows applications of Asymmetric Sharpless Aminohydroxylation (ASAH) in the stereoselective synthesis of vicinal amino alcohols as important intermediates in the total synthesis of complex molecules and natural products with significant biological activities.
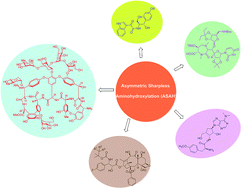
RSC Adv., 2018,8, 6634-6659
https://doi.org/10.1039/C7RA12625E
Stereoselective synthesis of 3-amino-2-oxindoles from isatin imines: new scaffolds for bioactivity evaluation
3-Substituted-3-aminooxindole motifs constitute the core structure of a number of natural products and drug candidates.
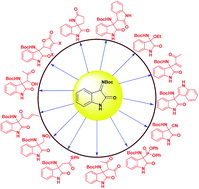
RSC Adv., 2015,5, 52481-52496
https://doi.org/10.1039/C5RA06969F
Recent development of direct asymmetric functionalization of inert C–H bonds
This review summarized recent development (contributions after 2009) in the area of direct catalytic asymmetric functionalization reactions of inert C–H bonds.
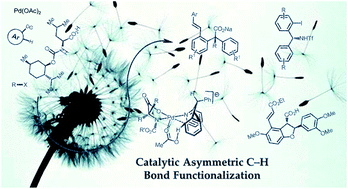
RSC Adv., 2014,4, 6173-6214
https://doi.org/10.1039/C3RA46996D
Catalytic asymmetric synthesis of 3-hydroxyoxindole : a potentially bioactive molecule
The recent emergence of biological activities of chiral 3-substituted-3-hydroxy-2-oxindoles has inspired synthetic chemists to develop new methodologies for their synthesis.
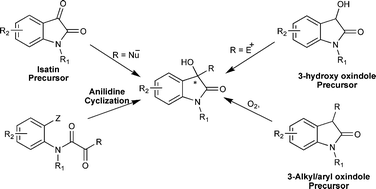
RSC Adv., 2012,2, 9748-9762
https://doi.org/10.1039/C2RA21131A
Asymmetric ring opening of racemic epoxides for enantioselective synthesis of (S)-β-amino alcohols by a cofactor self-sufficient cascade biocatalysis system
Asymmetric ring opening of racemic epoxides to enantiopure β-amino alcohols via a cascade biocatalysis system.
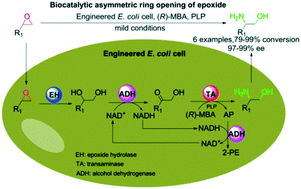
Catal. Sci. Technol., 2019,9, 70-74
https://doi.org/10.1039/C8CY02377H
Simple primary β-amino alcohols as organocatalysts for the asymmetric Michael addition of β-keto esters to nitroalkenes
Simple primary β-amino alcohols X act as an efficient organocatalysts in the asymmetric Michael addition of β-keto esters A with nitroalkenes B affording highly pure chiral Michael adducts C.
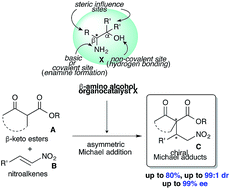
RSC Adv., 2021,11, 203-209
https://doi.org/10.1039/D0RA09041G
Enantioselective organocatalytic Michael reactions using chiral (R,R)-1,2-diphenylethylenediamine-derived thioureas
Although the Michael addition is a very well-known and widely applied reaction, cost-effective, metal-free, and readily prepared organic catalysts remain rare.

RSC Adv., 2020,10, 31808-31814
https://doi.org/10.1039/D0RA03550E
Insights into isothiourea-catalyzed asymmetric [3 + 3] annulation of α,β-unsaturated aryl esters with 2-acylbenzazoles: mechanism, origin of stereoselectivity and switchable chemoselectivity
The switchable chemoselectivity of isothiourea-catalyzed asymmetric [3 + 3] annulation of α,β-unsaturated aryl esters with 2-acylbenzazoles has been predicted successfully.
![Graphical abstract: Insights into isothiourea-catalyzed asymmetric [3 + 3] annulation of α,β-unsaturated aryl esters with 2-acylbenzazoles: mechanism, origin of stereoselectivity and switchable chemoselectivity](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D0CY00295J&imageInfo.ImageIdentifier.Year=2020)
Catal. Sci. Technol., 2020,10, 3664-3669
https://doi.org/10.1039/D0CY00295J
Simple organocatalyst component system for asymmetric hetero Diels–Alder reaction of isatins with enones
A simple two catalysts component system of β-amino alcohols (catalyst) and amino acids (co-catalyst) works as an efficient organocatalysts in hetero Diels–Alder reaction of isatins with enones to afford chiral spirooxindole-tetrahydropyranones.
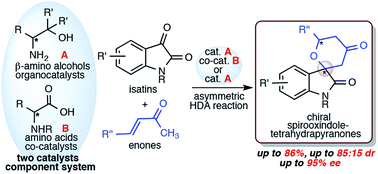
RSC Adv., 2020,10, 17486-17491
https://doi.org/10.1039/D0RA03006F
Development of an engineered thermostable amine dehydrogenase for the synthesis of structurally diverse chiral amines
Structurally diverse chiral amines and amino alcohols were synthesized using an engineered thermostable amine dehydrogenase, demonstrating its extensive synthesis potential.
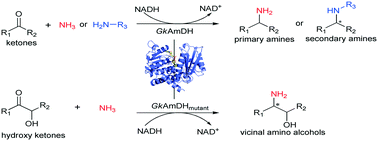
Catal. Sci. Technol., 2020,10, 2353-2358
https://doi.org/10.1039/D0CY00071J
Engineering an alcohol dehydrogenase with enhanced activity and stereoselectivity toward diaryl ketones: reduction of steric hindrance and change of the stereocontrol element
Engineering an alcohol dehydrogenase with enhanced activity and stereoselectivity toward diaryl ketones: reduction of steric hindrance and change of the stereocontrol element.

Catal. Sci. Technol., 2020,10, 1650-1660
https://doi.org/10.1039/C9CY02444A
Efficient asymmetric synthesis of chiral alcohols using high 2-propanol tolerance alcohol dehydrogenase SmADH2 via an environmentally friendly TBCR system
Based on substrate-coupled cofactor regeneration system, a high 2-propanol tolerance SmADH2 together with TBCR system can synthesise structurally diverse chiral alcohols at a high substrate loading with only 1.25 equivalents of 2-propanol.
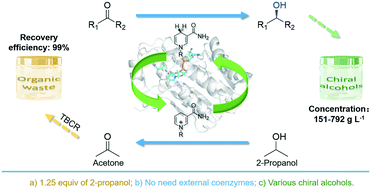
Catal. Sci. Technol., 2020,10, 70-78
https://doi.org/10.1039/C9CY01794A
Organocatalytic enantioselective conjugate addition of 2-naphthols to ortho-hydroxyphenyl substituted para-quinone methides: access to unsymmetrical triarylmethanes
The enantioselective conjugate addition of 2-naphthols to ortho-hydroxyphenyl substituted para-quinone methides has been achieved with the aid of a chiral phosphoric acid.

RSC Adv., 2019,9, 24212-24217
https://doi.org/10.1039/C9RA04768A
An effective strategy for creating asymmetric MOFs for chirality induction: a chiral Zr-based MOF for enantioselective epoxidation
A simple and rapid procedure was used to prepare chiral NU-1000 as a robust Zr-based MOF without complexity. The functionalization of NU-1000 was performed by utilizing chiral L-(+)-tartaric acid via solvent-assisted linker incorporation, resulting in [C-NU-1000]. A Mo-complex was immobilized onto chiral NU-1000 for enantioselective epoxidation.
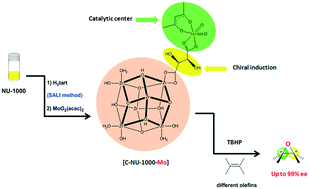
Catal. Sci. Technol., 2019,9, 3388-3397
https://doi.org/10.1039/C9CY00565J
Copper-catalysed enantioselective intramolecular etherification of propargylic esters: synthetic approach to chiral isochromans
The first example for direct synthesis of chiral terminal alkynyl isochromans.

RSC Adv., 2019,9, 18918-18922
https://doi.org/10.1039/C9RA03880A
Hydroxynitrile lyases covalently immobilized in continuous flow microreactors
Enzymes are supreme catalysts when it comes to high enantiopurities and their immobilization will pave the way for continuous operation.
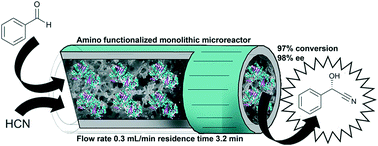
Catal. Sci. Technol., 2019,9, 1189-1200
https://doi.org/10.1039/C8CY02192A
Probing enantioselectivity in rhodium-catalyzed Si–C bond cleavage to construct silicon-stereocenters: a theoretical study
Density functional theory (DFT) calculations indicate that favorable oxidative addition/reductive elimination process from arylrhodium complex determines the enantioselectivity.
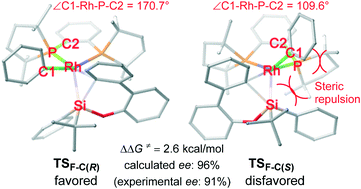
Catal. Sci. Technol., 2019,9, 646-651
https://doi.org/10.1039/C8CY02261E
Prediction on the origin of selectivities of NHC-catalyzed asymmetric dearomatization (CADA) reactions
The mechanism and origin of selectivities of NHC-catalyzed dearomatization reaction have been theoretically studied.

Catal. Sci. Technol., 2019,9, 465-476
https://doi.org/10.1039/C8CY02238K
Highly stereoselective spirocyclopropanation of various diazooxindoles with olefins catalyzed using Ru(II)-complex
Optically active spirocyclopropyloxindole derivatives were efficiently synthesized from diazooxindoles and olefins in the presence of a Ru(II)-Pheox catalyst.
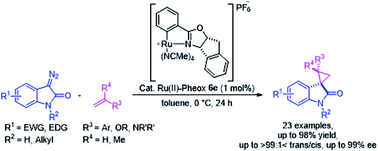
RSC Adv., 2018,8, 39865-39869
https://doi.org/10.1039/C8RA09212E
Asymmetric synthesis of chromene skeletons via organocatalytic domino reactions of in situ generated ortho-quinone methide with malononitrile and β-functionalized ketone
Enantioselective organocatalytic domino reactions of in situ generated ortho-quinone methides with malononitrile and β-functionalized ketones have been developed. This strategy could generate various chiral chromenes in high yields and stereoselectivities.
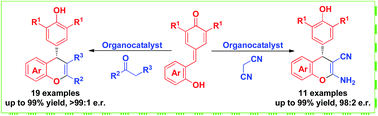
RSC Adv., 2017,7, 39216-39220
https://doi.org/10.1039/C7RA08157J
About this collection
We are very pleased to present our 10th Anniversary collection on asymmetric catalysis!Looking back over the last 10 years, we would like to showcase some of the very best articles that have been published in RSC Advances and Catalysis Science & Technology. Many of these papers have been cited hundreds of times, providing valuable advances for further research, and some continue to be among the journal’s most downloaded articles as of today.
We hope you enjoy our 10th Anniversary collection on asymmetric catalysis!