Highly stereoselective spirocyclopropanation of various diazooxindoles with olefins catalyzed using Ru(ii)-complex†
Abstract
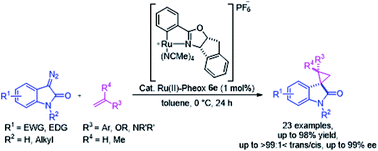
Optically active spirocyclopropyloxindole derivatives were efficiently synthesized from diazooxindoles and olefins in the presence of a Ru(II)-Pheox catalyst. Among a series of Ru(II)-Pheox catalysts, Ru(II)-Pheox 6e was determined to be the best catalyst for spirocyclopropanation reactions of diazooxindoles with various olefins in high yields (up to 98%) with high diastereoselectivities (up to trans:cis = >99:1<) and enantioselectivities (up to 99% ee). Furthermore, as the first catalytic asymmetric synthesis, anti-HIV active candidate 4a and a bioactive compound of AMPK modulator 4c were easily synthesized from the corresponding diazooxindoles 1i and 1b, respectively, in high yields with high enantioselectivities (4a: 82% yield, 95% ee, 4b: 99% yield, 93% ee).
- This article is part of the themed collection: Asymmetric catalysis


 Please wait while we load your content...
Please wait while we load your content...