Development of an engineered thermostable amine dehydrogenase for the synthesis of structurally diverse chiral amines†
Abstract
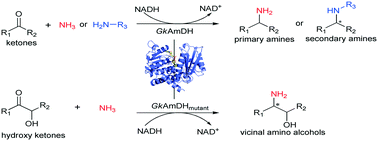
Amine dehydrogenases (AmDHs) are emerging as a class of attractive biocatalysts for synthesizing chiral amines via asymmetric reductive amination of ketones with inexpensive ammonia as an amino donor. However, the AmDHs developed to date exhibit limited substrate scope. Here, using directed evolution, we engineered a GkAmDH based on a thermostable phenylalanine dehydrogenase from Geobacillus kaustophilus. The newly developed AmDH is able to catalyze reductive amination of a diverse set of ketones and functionalized hydroxy ketones with ammonia or primary amines with up to >99% conversion, thus accessing structurally diverse chiral primary and secondary amines and chiral vicinal amino alcohols, with excellent enantioselectivity (up to >99% ee) and releasing water as the sole by-product.
- This article is part of the themed collection: Asymmetric catalysis


 Please wait while we load your content...
Please wait while we load your content...