Asymmetric catalysis in direct nitromethane-free Henry reactions
Abstract
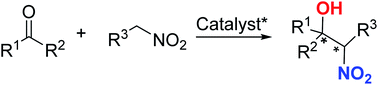
A great number of reports have described asymmetric catalytic Henry reactions using nitromethanes as pronucleophiles, but far more challenging is diastereoselective catalytic Henry reactions using substituted higher nitroalkanes instead of nitromethane to generate chiral β-nitro alcohol scaffolds with four adjacent stereogenic centers in a one-pot operation. This review summarizes the current state and applications of such reactions involving complex nitroalkane coupling with various carbonyl compounds for resolving double chiral centers with high enantio- and diastereoselectivities.
- This article is part of the themed collections: Asymmetric catalysis and 2020 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...