Themed collection Chirality

Transfer of chirality from ligands to metal centers: recent examples
The recent advances of chirality transfer in complexes are described with a special focus on applications in materials science.
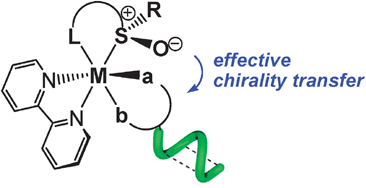
Chem. Commun., 2012,48, 9687-9695
https://doi.org/10.1039/C2CC31542D
Asymmetric transformations involving 1,2-dicarbonyl compounds as pronucleophiles
This article concentrates on the versatile nucleophilic reactivity of 1,2-dicarbonyl compounds in various asymmetric transformations.
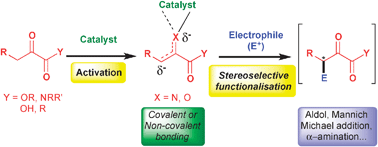
Chem. Commun., 2012,48, 6763-6775
https://doi.org/10.1039/C2CC30691C
Point-to-helical chirality transfer for a scalable and resolution-free synthesis of a helicenoidal DMAP organocatalyst
The synthesis of a second-generation [6]-helicenoidal DMAP organocatalyst is reported.
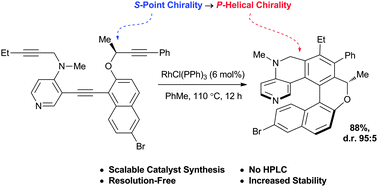
Chem. Commun., 2012,48, 11181-11183
https://doi.org/10.1039/C2CC35583C
Asymmetric autocatalysis initiated by achiral nucleic acid base adenine : implications on the origin of homochirality of biomolecules
Enantiomorphous adeninium dinitrate crystals acted as the source of chirality in asymmetric autocatalysis to produce (S)- and (R)-alkanols.
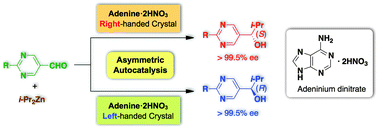
Chem. Commun., 2012,48, 10538-10540
https://doi.org/10.1039/C2CC34928K
Chiral recognition in metal–organic frameworks studied by solid-state NMR spectroscopy using chiral solvating agents
The use of a chiral solvating agent (TFPE) for chiral recognition in functionalized metal–organic frameworks by solid-state 13C NMR spectroscopy is demonstrated for the first time.
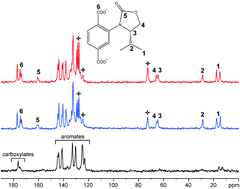
Chem. Commun., 2012,48, 10484-10486
https://doi.org/10.1039/C2CC35366K
Chiral assembly of dodecahedral cavities into porous metal–organic frameworks
Presented here is the corner-sharing assembly of dodecahedral cavities with both paddle-wheel [M2(CO2)4] units and trimeric [M3(μ3-O)(CO2)6] units into two isomorphous chiral microporous metal–organic frameworks.
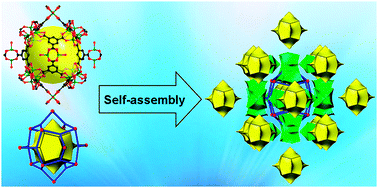
Chem. Commun., 2012,48, 9424-9426
https://doi.org/10.1039/C2CC35024F
Chiral recognition in aggregation of gold nanoparticles grafted with helicenes
Chiral recognition was affected by helicene stereoisomerism on the surface of gold nanoparticles in their aggregation.
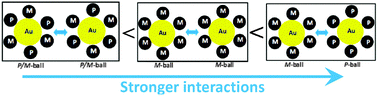
Chem. Commun., 2012,48, 7383-7385
https://doi.org/10.1039/C2CC32735J
Unexpected chiral induction from achiral cationic polythiophene aggregates and its application to the sugar pattern recognition
A polythiophene derivative forms chiral aggregates influenced by added sugars, the ICD intensity being correlated with the specific optical rotation.
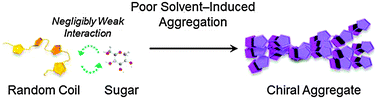
Chem. Commun., 2012,48, 7091-7093
https://doi.org/10.1039/C2CC33162D
A homochiral metal–organic framework membrane for enantioselective separation
For the first time, a homochiral MOF membrane was prepared for the enantioselective separation of important chiral compounds.
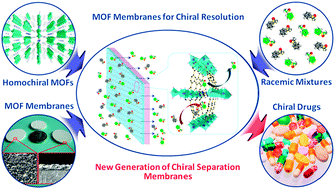
Chem. Commun., 2012,48, 7022-7024
https://doi.org/10.1039/C2CC32595K
Enantio- and diastereoselective hetero-Diels–Alder reactions between 2-aza-3-silyloxy-1,3-butadienes and aldehydes catalyzed by chiral dirhodium(II) carboxamidates
The first catalytic asymmetric hetero-Diels–Alder reaction between 2-aza-3-silyloxy-1,3-butadienes and aldehydes is described. With Rh2(S-BPTPI)4, the cycloaddition reaction gave all-cis-substituted 1,3-oxazinan-4-ones in high yields with up to 98% ee.
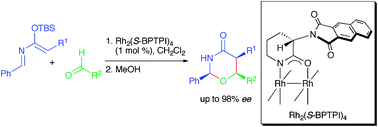
Chem. Commun., 2012,48, 6969-6971
https://doi.org/10.1039/C2CC32876C
Chiral nematic organo-siloxane oligopodes based on an axially chiral binaphthalene core
The synthesis of a novel class of organosiloxane oligopodes, based on an axially chiral binaphthalene core is described and their mesogenic properties are fully characterised.

Chem. Commun., 2012,48, 6851-6853
https://doi.org/10.1039/C2CC33050D
Chiral metal–organic frameworks with tunable open channels as single-site asymmetric cyclopropanation catalysts
We have synthesized a new pair of porous chiral MOFs that were constructed from the same catalytically active bridging ligand but possessed different open channel sizes as a result of the different catenation modes.
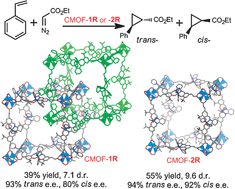
Chem. Commun., 2012,48, 6508-6510
https://doi.org/10.1039/C2CC32232C
Reversible helix–random coil transition of poly(m-phenylenediethynylene) by a rotaxane switch
Pendant rotaxane switch-tethering poly(m-phenylene diethynylene) was synthesized by polyoxidative coupling of a rotaxane containing an axle-terminal m-diethynylbenzene group and an optically active crown ether. The reversible helix–random coil transition of the polymer was successfully performed by the positional switching of the rotaxane wheel.
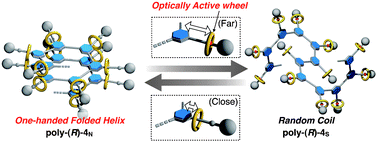
Chem. Commun., 2012,48, 6478-6480
https://doi.org/10.1039/C2CC18116A
Construction of supramolecular helical nanofibers using renewable biomaterials: self-assembly of a cytidylic acid-appended bolaamphiphile in lemon juice
Left-handed helical nanofibers were produced from the self-assembly of nucleotide-appended bola-shape molecules in lemon juice.
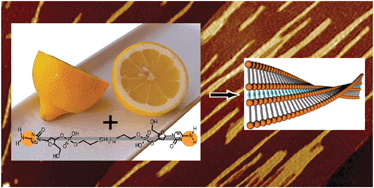
Chem. Commun., 2012,48, 6633-6635
https://doi.org/10.1039/C2CC17787K
Chiroptical generation and inversion during the mirror-symmetry-breaking aggregation of dialkylpolysilanes due to limonene chirality
The emerging and chiroptical switching during the mirror-symmetry-breaking aggregation of optically inactive polysilanes due to solvent limonene chirality were found.
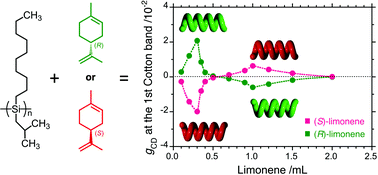
Chem. Commun., 2012,48, 6636-6638
https://doi.org/10.1039/C2CC17845A
Solvent dependence of helix stability in aromatic oligoamide foldamers
Monitoring racemization allows us to assess helix stability, which is highest in protic solvents.

Chem. Commun., 2012,48, 6337-6339
https://doi.org/10.1039/C2CC31533E
Enantioselective catalysis with a chiral, phosphane -containing PMO material
Rigidly bridged BINAP/biphenylene co-condensed periodic mesoporous organosilica was synthesized and showed crystal-like wall structure and high activity in enantioselective hydrogenations.
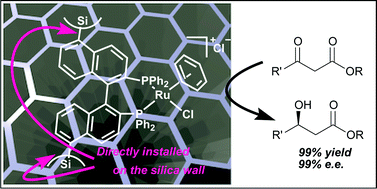
Chem. Commun., 2012,48, 6369-6371
https://doi.org/10.1039/C2CC31247F
Absolute chirality of the γ-polymorph of glycine : correlation of the absolute structure with the optical rotation
Crystal specimens of the γ-polymorph of achiral glycine which crystallize in space groups P31 and P32 as determined by the anomalous X-ray scattering method are shown to be laevorotatory and dextrorotatory, respectively, as determined by optical rotation of the crystals.
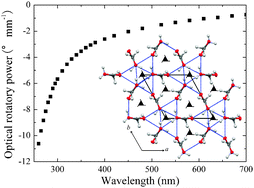
Chem. Commun., 2012,48, 6031-6033
https://doi.org/10.1039/C2CC30549F
Amplification of enantioselectivity and sensitivity based on non-linear response of molecular wire bearing pseudo-18-crown-6 to chiral amines
An intelligent chiral chemosensor capable of chirality amplification was designed and constructed as poly(phenyleneethynylene) having chiral pseudo-18-crown-6. Both sensitivity and selectivity were amplified from those limited by the law of mass action.

Chem. Commun., 2012,48, 6052-6054
https://doi.org/10.1039/C2CC30417A
On the abundance of chiral crystals
We propose that there is a vast ocean of chiral crystals just waiting to be discovered/proclaimed.
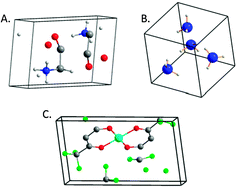
Chem. Commun., 2012,48, 5874-5876
https://doi.org/10.1039/C2CC17727G
Enantioselective Friedel–Crafts reactions between phenols and N-tosylaldimines catalyzed by a leucine-derived bifunctional catalyst
Chiral benzylic amines were obtained through the Friedel–Crafts reactions between phenols and N-tosylaldimines catalyzed by a leucine-derived catalyst.
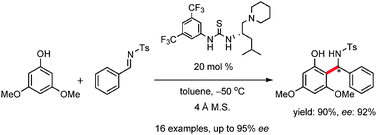
Chem. Commun., 2012,48, 5518-5520
https://doi.org/10.1039/C2CC31735D
Asymmetric aldol reaction via memory of chirality
Asymmetric aldol reactions take place via axially chiral enolate intermediates to give chiral oxazolidones with a tetrasubstituted chiral center.

Chem. Commun., 2012,48, 5346-5348
https://doi.org/10.1039/C2CC31447A
Kinetic effects of tartaric acid on the growth of chiral J-aggregates of tetrakis(4-sulfonatophenyl)porphyrin
The kinetics of growth for chiral J-aggregates of H4TPPS4 porphyrin have been investigated under different experimental conditions in the presence of tartaric acid. The observed rate constants and the anisotropy factor g show a defined dependence on the enantiomer used as a chiral templating agent.
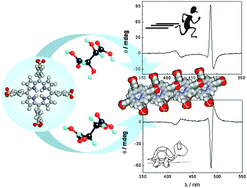
Chem. Commun., 2012,48, 4872-4874
https://doi.org/10.1039/C2CC00028H
Highly sensitive determination of enantiomeric composition of chiral acids based on aggregation-induced emission
A new chiral tetraphenylethylene derivative with the AIE effect was synthesized and showed not only high enantioselectivity for a wide range of chiral acids but also a high sensitivity of 10−6 M scale. The enantiomeric purity of chiral acids could be quantitatively determined by this chiral sensor.
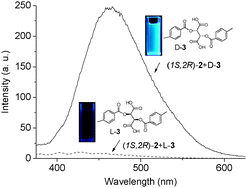
Chem. Commun., 2012,48, 4908-4910
https://doi.org/10.1039/C2CC30448A
Molecular chirality and chiral capsule-type dimer formation of cyclic triamides via hydrogen-bonding interactions
Chiral separation of cyclic triamide 4a was achieved by simple crystallization to afford chiral capsule-type dimer structure.
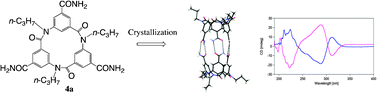
Chem. Commun., 2012,48, 4809-4811
https://doi.org/10.1039/C2CC18177K
Pseudo helix-sense-selective polymerisation of achiral substituted acetylenes
Pseudo helix-sense-selective polymerization of achiral substituted acetylenes having dynamic covalent bonds to yield static one-handed helical polymers has been achieved.
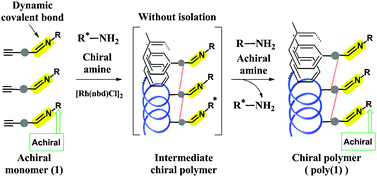
Chem. Commun., 2012,48, 4761-4763
https://doi.org/10.1039/C2CC00030J
Irreversible visual sensing of humidity using a cholesteric liquid crystal
Humidity sensing by hydrolysis of a chiral dopant in a cholesteric liquid crystal leading to irreversible color change is demonstrated.
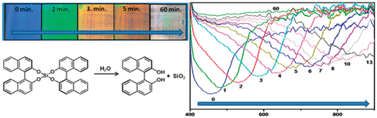
Chem. Commun., 2012,48, 4579-4581
https://doi.org/10.1039/C2CC16934G
Ligand dependence of the synthetic approach and chiroptical properties of a magic cluster protected with a bicyclic chiral thiolate
Camphorthiolate-protected Au25 clusters were synthesised. The optical activity is traced to the chirality of the ligands and is dominated by their orientation rather than their internal structure.
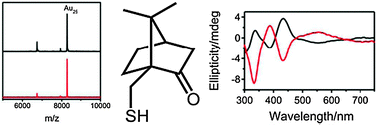
Chem. Commun., 2012,48, 4630-4632
https://doi.org/10.1039/C2CC00056C
Twists and turns in the hierarchical self-assembly pathways of a non-amphiphilic chiral supramolecular material
A variety of different chiral forms, including unprecedented croissants, are self-assembled from helical supramolecular fibres by varying the processing conditions.

Chem. Commun., 2012,48, 4552-4554
https://doi.org/10.1039/C2CC30789H
Unexpected stereomutation dependence on the chemical structure of helical vinyl glycopolymers
A small chemical structure change causes a remarkable influence on the stereomutational rate of helical vinyl glycopolymers.
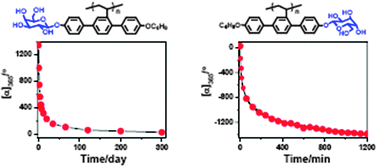
Chem. Commun., 2012,48, 4341-4343
https://doi.org/10.1039/C2CC00036A
Induction of chirality in porphyrin –(bis)calixarene assemblies: a mixed covalent–non-covalent vs a fully non-covalent approach
Two strategies for the syntheses of chiral porphyrin–(bis)calixarene multi-component supramolecular assemblies are reported.
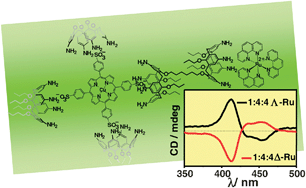
Chem. Commun., 2012,48, 4046-4048
https://doi.org/10.1039/C2CC30366C
Design of main-chain polymers of chiral imidazolidinone for asymmetric organocatalysis application
Main-chain polymers of chiral imidazolidinone are useful as polymeric chiral organocatalysts for the asymmetric Diels–Alder reaction of cyclopentadiene and cinnamaldehyde.
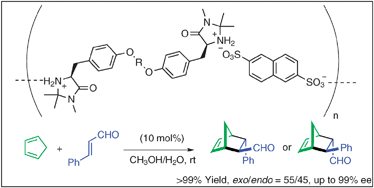
Chem. Commun., 2012,48, 4011-4013
https://doi.org/10.1039/C2CC18115K
Modular synthesis of optically active lactones by Ru-catalyzed asymmetric allylic carboxylation and ring-closing metathesis reaction
A new synthetic route to chiral γ- and δ-lactones has been demonstrated via asymmetric allylic carboxylation with a planar-chiral Cp′Ru catalyst and RCM.
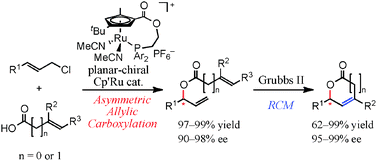
Chem. Commun., 2012,48, 3872-3874
https://doi.org/10.1039/C2CC18137A
Promotion effects of optical antipodes on the formation of helical fibrils: chiral perfluorinated gelators
Coiled fibrils are preferably formed by perfluorinated gelators when an optical antipode is added to the surplus amount of chiral aggregates, while crystals are formed immediately from the pure enantiomer form.

Chem. Commun., 2012,48, 3860-3862
https://doi.org/10.1039/C2CC18164A
A stereoselectively deuterated supramolecular motif to probe the role of solvent during self-assembly processes
The structure of the alkane solvent strongly affects the expression of the supramolecular chirality in stereoselectively deuterated benzene-1,3,5-tricarboxamides.
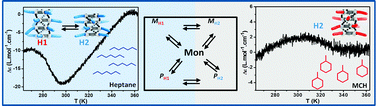
Chem. Commun., 2012,48, 3803-3805
https://doi.org/10.1039/C2CC17284D
Helicity inversion from left- to right-handed square planar Pd(II) complexes: synthesis of a diastereomer pair from a single chiral ligand and their structure dynamism
A diastereomer pair of left- and right-handed square planar Pd(II) complexes was synthesized from a single chiral ligand as kinetic and thermodynamic products. Helicity inversion between the diastereomers occurred rapidly under thermal and microwave irradiation conditions.
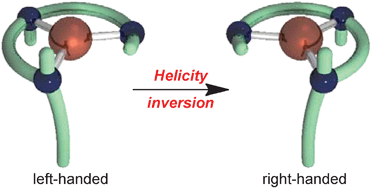
Chem. Commun., 2012,48, 3721-3723
https://doi.org/10.1039/C2CC18154A
Asymmetric synthesis of α-amino boronate esters via organocatalytic pinacolboryl addition to tosylaldimines
A simple one-pot transformation from tosylaldimines towards chiral 1,2-amino alcohols by enantioselective metal free boryl addition to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N followed by homologation/oxidation.
N followed by homologation/oxidation.
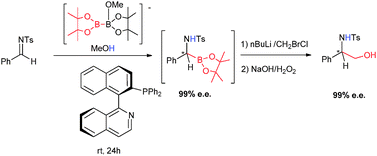
Chem. Commun., 2012,48, 3769-3771
https://doi.org/10.1039/C2CC00020B
Helical phase from blending of chiral block copolymer and homopolymer
A helical phase is formed in the blends of chiral block copolymer due to an enhancement of helical steric hindrance.
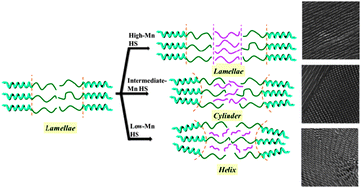
Chem. Commun., 2012,48, 3665-3667
https://doi.org/10.1039/C2CC18163K
Enantioenrichment in sublimed amino acid mixtures
Enantioenrichment in two-component amino acid mixtures is obtained after sublimation, provided that one converts into a conglomerate phase.
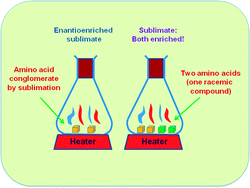
Chem. Commun., 2012,48, 3623-3625
https://doi.org/10.1039/C2CC18129K
Induced mirror symmetry breaking via template-controlled copolymerization : theoretical insights
Calculated ee values of the peptide chains of each chain length l for three starting concentrations (in moles) of the host and guest monomers rtot : r′tot : stot : s′tot.
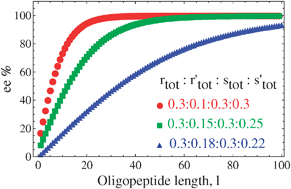
Chem. Commun., 2012,48, 3659-3661
https://doi.org/10.1039/C2CC18045F
Efficient dynamic kinetic resolution of racemic secondary alcohols by a chemoenzymatic system using bifunctional iridium complexes with C–N chelate amido ligands
DKR of racemic secondary alcohols was achieved efficiently by the combined catalyst system of bifunctional amidoiridium complexes with CALB.
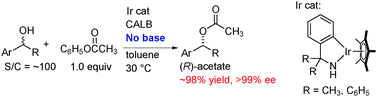
Chem. Commun., 2012,48, 3635-3637
https://doi.org/10.1039/C2CC30333G
A triple helix of double helicates: three hierarchical levels of self-assembly in a single structure
A dinuclear double helical complex [Ag2L2]2+ assembles into infinite chains via Ag⋯Ag interactions; three such chains form a triple helical braid in the crystal which therefore displays three distinct levels of supramolecular organisation.
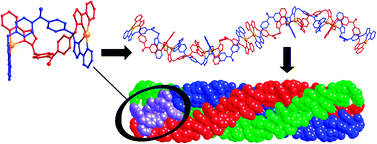
Chem. Commun., 2012,48, 3605-3607
https://doi.org/10.1039/C2CC30197K
Copper(II)/phenanthroline -mediated CD-enhancement and chiral memory effect on a meta-ethynylpyridine oligomer
meta-Ethynylpyridine 18-mer associates with octyl β-D-glucopyranoside to show an induced circular dichroism (ICD) band around 337 nm in CH2Cl2. The ICD was enhanced and memorized by the addition of copper(II) triflate and o-phenanthroline.
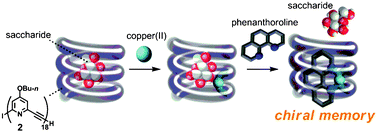
Chem. Commun., 2012,48, 3330-3332
https://doi.org/10.1039/C2CC00063F
Helical polymer brushes with a preferred-handed helix-sense triggered by a terminal optically active group in the pendant
Helical polymer brushes bearing a polyacetylene backbone and polyisocyanate pendants were synthesized, whose helix-sense was controlled by the chiral domino effect.
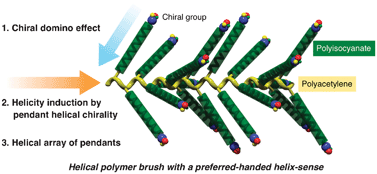
Chem. Commun., 2012,48, 3342-3344
https://doi.org/10.1039/C2CC00024E
Chirality induction in metal-induced achiral polythiophene aggregates assisted by optically active amines and polythiophene
An achiral polythiophene formed a unique optically active metal-induced supramolecular aggregate upon complexation with chiral amines or in the presence of a chiral polythiophene in a good solvent for the polymers, thus showing an induced circular dichroism.
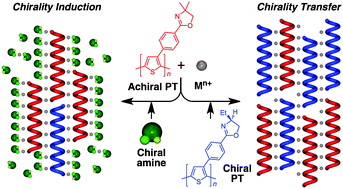
Chem. Commun., 2012,48, 3291-3293
https://doi.org/10.1039/C2CC17940G
Giving substance to the Losanitsch series
A series of oligoparaxylenes with multiple axes of chirality was synthesized and their atropisomers were found by variable temperature 1H NMR spectroscopy to obey the Losanitsch series.

Chem. Commun., 2012,48, 3158-3160
https://doi.org/10.1039/C2CC17734J
The dynamic chromatographic behavior of tri-o-thymotide on HPLC chiral stationary phases
The interconverting stereoisomers of tri-o-thymotide have been separated by HPLC on chiral stationary phases and the dynamic chromatographic patterns interpreted in terms of exchange between enantiomeric helical and propeller conformations.
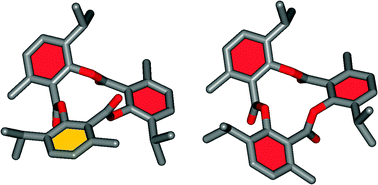
Chem. Commun., 2012,48, 3167-3169
https://doi.org/10.1039/C2CC00021K
Synthesis of chiral ionic liquids by ion cross-metathesis : en route to enantioselective water–ionic liquid extraction (EWILE), an eco-friendly variant of the ELLE process
From two initial ILs two other ILs are obtained by simultaneous ion exchange giving access to an eco-friendly ELLE process.
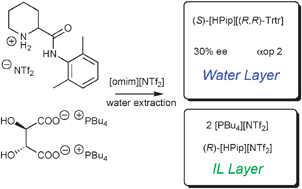
Chem. Commun., 2012,48, 3185-3187
https://doi.org/10.1039/C2CC17799D
A novel C-5′ substituted cinchona alkaloid -derived catalyst promotes additions of alkyl thiols to nitroolefins with excellent enantioselectivity
A new bifunctional C-5′ substituted cinchona alkaloid-based catalyst promotes the first highly enantioselective additions of alkyl thiols to nitrostyrenes.
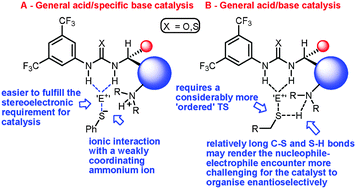
Chem. Commun., 2012,48, 2849-2851
https://doi.org/10.1039/C2CC17965B
Occurrence of spontaneous resolution of ketoprofen with a racemic crystal structure by simple crystallization under nonequilibrium preferential enrichment conditions
Nearly racemic ketoprofen was spontaneously resolved into the two enantiomers by simple crystallization under nonequilibrium preferential enrichment conditions.
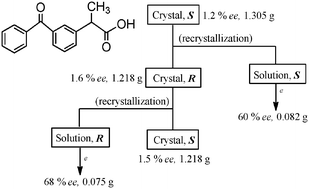
Chem. Commun., 2012,48, 2791-2793
https://doi.org/10.1039/C2CC18132K
Diastereoselective indium-mediated allylation of N-tert-butanesulfinyl ketimines : easy access to asymmetric quaternary stereocenters bearing nitrogen atoms
Indium-mediated allylation of N-tert-butanesulfinyl ketimines afforded in high yields and diastereoselectivities homoallylic amine derivatives with the nitrogen atom bonded to a quaternary stereocenter.
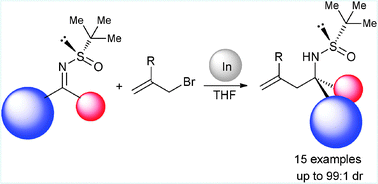
Chem. Commun., 2012,48, 2543-2545
https://doi.org/10.1039/C2CC17493F
One-pot Crabbé homologation-radical cascade cyclisation with memory of chirality
The tandem Crabbé homologation-radical rearrangement of terminal enediynes leads, in a one-pot procedure, to the enantioselective synthesis of six- and seven-membered ring α-aminoesters bearing a quaternary stereocenter based on the phenomenon of memory of chirality.
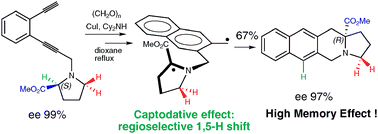
Chem. Commun., 2012,48, 2549-2551
https://doi.org/10.1039/C2CC17830C
Ligand denticity controls enantiomeric preference in DNA-based asymmetric catalysis
The enantiomeric outcome of DNA-based catalytic reactions is controlled by the denticity of the ligand coordinated to the Cu(II) ion.
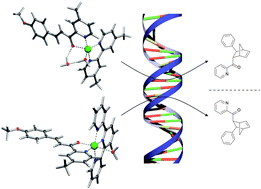
Chem. Commun., 2012,48, 2394-2396
https://doi.org/10.1039/C2CC17350F
Asymmetric assembly of 2-oxindole and α-angelica lactone units to construct vicinal quaternary chiral centers
The first Lewis base-catalysed asymmetric assembly of α-angelica lactone and Morita–Baylis–Hillman carbonates of isatins has been developed.
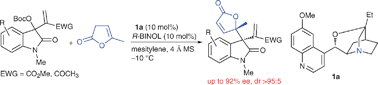
Chem. Commun., 2012,48, 2439-2441
https://doi.org/10.1039/C2CC17777C
Mirror symmetry breaking and chiral amplification in foldamer-based supramolecular helical aggregates
A supramolecular growth process based on secondary nucleation controls the chiral amplification of a lock-washer shaped foldamer.
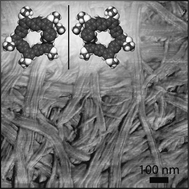
Chem. Commun., 2012,48, 2292-2294
https://doi.org/10.1039/C2CC16266K
A new strategy for chiral recognition of amino acids
A new strategy is established for detecting chiral amino acids based on the electron transfer from hemoglobin Fe(II) to Cu(II) in copper complexes of the amino acids.
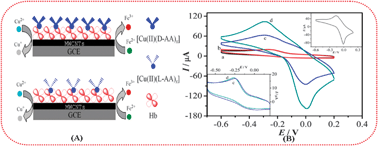
Chem. Commun., 2012,48, 2322-2324
https://doi.org/10.1039/C2CC17301H
Near-infrared chiro-optical effects in metallogels
Robust chiral gels were obtained from alkane solutions with rationally designed nickel–bis(dithiolene) complexes appended with cholesteryl fragments.
Chem. Commun., 2012,48, 2283-2285
https://doi.org/10.1039/C2CC16549J
Highly enantioselective Mukaiyama aldol reaction in aqueous conditions using a chiral iron(II) bipyridine catalyst
A highly enantioselective catalytic Mukaiyama aldol reaction of silyl enol ethers with aldehydes in aqueous conditions is described using a chiral FeII–bipyridine complex.
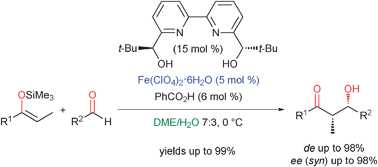
Chem. Commun., 2012,48, 2289-2291
https://doi.org/10.1039/C1CC16409K
Right- and left-handedness of 21 symmetrical herringbone assemblies of benzene
A universal method to determine handedness of 21 helical assemblies composed of planar aromatic molecules is proposed.
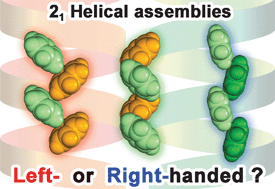
Chem. Commun., 2012,48, 2219-2221
https://doi.org/10.1039/C2CC17719F
Cu-catalyzed asymmetric [3+2] cycloaddition of α-iminoamides with activated olefins
A variety of 2-amido pyrrolidines, including Weinreb-type amides, have been prepared with very high exo diastereoselectivity and enantioselectivitiy in the reaction of α-iminoamides with activated alkenes.
![Graphical abstract: Cu-catalyzed asymmetric [3+2] cycloaddition of α-iminoamides with activated olefins](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C2CC17149J&imageInfo.ImageIdentifier.Year=2012)
Chem. Commun., 2012,48, 2149-2151
https://doi.org/10.1039/C2CC17149J
Molecular chirality induction to an achiral π-conjugated polymer by circularly polarized light
A preferred-handed helical conformation was reversibly induced to poly(9,9-dioctylfluoren-2,7-diyl) upon irradiation by circularly polarized light as the only source of chirality.
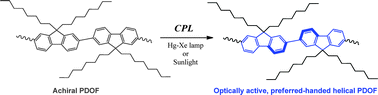
Chem. Commun., 2012,48, 1871-1873
https://doi.org/10.1039/C2CC17027B
About this collection
This ChemComm web theme issue showcases cutting-edge research in the field of chiral chemistry. The articles will include contributions on asymmetric synthesis, chiral recognition and separation, chiral assembly, supramolecular chirality, foldamers, materials, chiroptical spectroscopy, and the origins and emergence of chirality.
The guest editors for this issue are David Amabilino (Barcelona Materials Science Institute, CSIC) and Eiji Yashima (Nagoya University).
Articles in this web themed issue will be added below as soon as possible after they are published. Please return to this page frequently to see the collection grow.