Synthesis of chiral ionic liquids by ion cross-metathesis: en route to enantioselective water–ionic liquidextraction (EWILE), an eco-friendly variant of the ELLE process†‡
Abstract
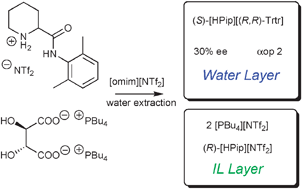
From two initial ILs two other ILs are obtained by simultaneous ion exchange. The hydrophobic ions unite in the hydrophobic layer, whereas the hydrophilic ions gather in
- This article is part of the themed collection: Chirality

 Please wait while we load your content...
Please wait while we load your content...