Themed collection Polycyclic Reactions in Synthesis and Biosynthesis

β-Amyrin biosynthesis: catalytic mechanism and substrate recognition
In the past five years, there have been remarkable advances in the study of β-amyrin synthase. This review outlines the catalytic mechanism and substrate recognition in β-amyrin biosynthesis, which have been attained by the site-directed mutagenesis and substrate analog experiments.
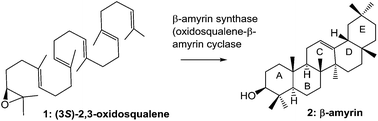
Org. Biomol. Chem., 2017,15, 2869-2891
https://doi.org/10.1039/C7OB00238F
Computational studies on the cyclization of squalene to the steroids and hopenes
A review of computational studies of the related biosyntheses of steroids and hopenes reported during the last two decades is presented.
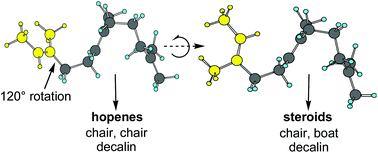
Org. Biomol. Chem., 2017,15, 2133-2145
https://doi.org/10.1039/C7OB00222J
cis or trans with class II diterpene cyclases
Isoprenoid precursors readily undergo (poly)cyclization in electrophilic reaction cascades, presumably as internal addition of the carbon–carbon double-bonds from neighboring isoprenyl repeats readily forms relatively stable cyclohexyl tertiary carbocation intermediates.
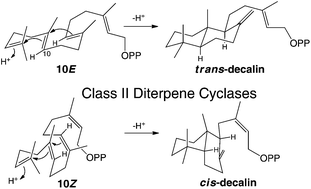
Org. Biomol. Chem., 2017,15, 3158-3160
https://doi.org/10.1039/C7OB00510E
Enantioselective Diels-Alder-lactamization organocascades employing a furan-based diene
α,β-Unsaturated acylammonium salts are useful dienophiles enabling highly enantioselective and stereodivergent Diels-Alder-initiated organocascades with furan-based dienes.
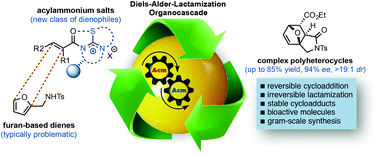
Org. Biomol. Chem., 2017,15, 3179-3183
https://doi.org/10.1039/C6OB02738E
Isoafricanol synthase from Streptomyces malaysiensis
A terpene cyclases from Streptomyces malaysiensis was characterised as (+)-isoafricanol synthase and its mechanism was investigated using isotopically labelled substrates.
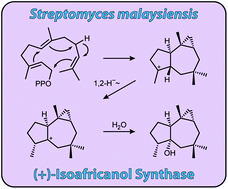
Org. Biomol. Chem., 2017,15, 2353-2358
https://doi.org/10.1039/C7OB00234C
Broad scope gold(I)-catalysed polyenyne cyclisations for the formation of up to four carbon–carbon bonds
The polycyclisation of polyeneynes catalyzed by gold(I) has been extended for the first time to the simultaneous formation of up to four carbon–carbon bonds, leading to steroid-like molecules with high stereoselectivity in a single step with low catalyst loadings.
Org. Biomol. Chem., 2017,15, 2163-2167
https://doi.org/10.1039/C7OB00235A
Cationic polycyclization of ynamides: building up molecular complexity
Simple activation of readily available ynamides under acidic conditions triggers an unprecedented cationic polycyclization yielding highly substituted polycyclic nitrogen heterocycles.
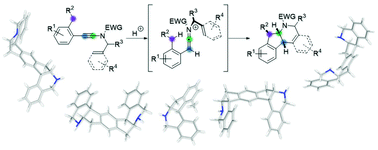
Org. Biomol. Chem., 2017,15, 4399-4416
https://doi.org/10.1039/C7OB00850C
Selective construction of quaternary stereocentres in radical cyclisation cascades triggered by electron-transfer reduction of amide-type carbonyls
Radical cyclisation cascades triggered by electron-transfer to amide-type carbonyls using SmI2–H2O–LiBr, result in the selective construction of quaternary carbon stereocentres.
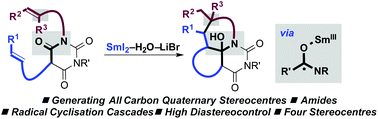
Org. Biomol. Chem., 2017,15, 4159-4164
https://doi.org/10.1039/C7OB00739F
Photochemical studies on bis-sulfide and -sulfone tethered polyenic derivatives
This study focusses on the [2 + 2]-photocycloaddition of a symmetric polyenic system tethered by an aryl bis-sulfide or sulfone platform.
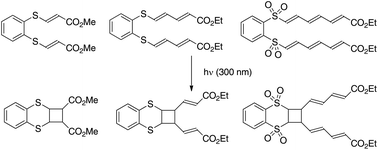
Org. Biomol. Chem., 2017,15, 4180-4190
https://doi.org/10.1039/C7OB00551B
Changing the path of least resistance, or access to endo-dig products via a sequence of three exo-trig transition states: electronic effects in homoallyic ring expansion cascades of alkenyl isonitriles
Substituent effects reshape the potential energy surfaces for radical homoallylic expansion/fragmentation cascades that transform alkenyl isonitriles into N-heteroaromatics
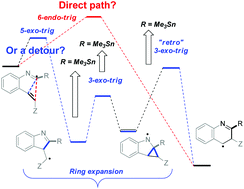
Org. Biomol. Chem., 2017,15, 4135-4143
https://doi.org/10.1039/C7OB00527J
Stereochemical investigations of the Tetrahymena cyclase, a model system for euphane/tirucallane biosynthesis
The squalene cyclase of Tetrahymena pyriformis cyclizes 2,3-dihydrosqualene to euph-7-ene; after truncation of the substrate, “tirucall-7-enes” are formed.
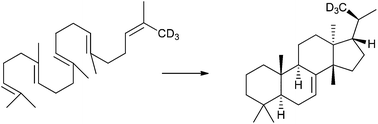
Org. Biomol. Chem., 2017,15, 2823-2830
https://doi.org/10.1039/C7OB00296C
Viability of dodecahedrane-forming radical polycyclizations
The results of density functional theory calculations on thiyl radical-promoted polycyclization to form dodecahedrane are described.
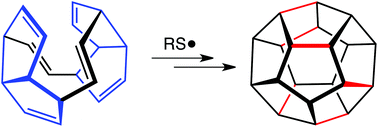
Org. Biomol. Chem., 2017,15, 1976-1979
https://doi.org/10.1039/C6OB02300B
Gold(I)-catalyzed addition of aldehydes to cyclopropylidene bearing 6-aryl-1,5-enynes
A diastereoselective, gold-catalyzed cascading cycloisomerization of alkylidene cyclopropane bearing 1,5-enynes that terminates in a cyclo-addition of aldehydes has been developed.
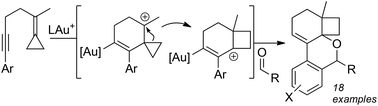
Org. Biomol. Chem., 2016,14, 11261-11265
https://doi.org/10.1039/C6OB02128J
About this collection
This themed collection, Guest Edited by Professors Dean Tantillo, Michel Gagné, and Rong-Jie Chein, covers research on the development of new polycyclization reactions including new reagents and catalysts, applications to total synthesis and/or diversity-oriented synthesis, and experimental and/or theoretical mechanistic studies on polycyclizations in synthesis or biosynthesis.
Articles in this themed collection will be added below as soon as possible after they are published.
Please return to this page frequently to see the collection grow.