Changing the path of least resistance, or access to endo-dig products via a sequence of three exo-trig transition states: electronic effects in homoallyic ring expansion cascades of alkenyl isonitriles†
Abstract
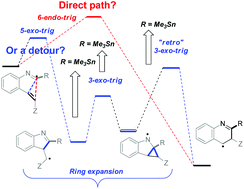
Chemoselective addition of radicals to isonitriles can be harnessed for initiating reaction cascades designed to overcome the stereoelectronic restrictions on homoallylic ring expansion in alkyne reactions and to develop a new general route for the preparation of N-heteroaromatics. This method utilizes alkenes as synthetic equivalents of alkynes by coupling homoallylic ring expansion to yield the formal “6-endo” products with aromatization via stereoelectronically assisted C–C bond scission. Detailed computational analysis of the individual steps of the homoallylic expansion sequence maps effects of substituents and structural constraints on this multi-step potential energy surface.
- This article is part of the themed collection: Polycyclic Reactions in Synthesis and Biosynthesis


 Please wait while we load your content...
Please wait while we load your content...