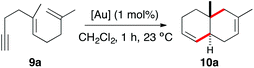Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Broad scope gold(I)-catalysed polyenyne cyclisations for the formation of up to four carbon–carbon bonds†
Zhouting
Rong
a and
Antonio M.
Echavarren
 *ab
*ab
aInstitute of Chemical Research of Catalonia (ICIQ), Barcelona Institute of Science and Technology, Av. Països Catalans 16, 43007 Tarragona, Spain. E-mail: aechavarren@iciq.es
bDepartament de QuímicaAnalítica i QuímicaOrgànica, UniversitatRovira i Virgili, C/ Marcel·li Domingo s/n, 43007 Tarragona, Spain
First published on 13th February 2017
Abstract
The polycyclisation of polyenynes catalyzed by gold(I) has been extended for the first time to the simultaneous formation of up to four carbon–carbon bonds, leading to steroid-like molecules with high stereoselectivity in a single step with low catalyst loadings. In addition to terminal alkynes, bromoalkynes can also be used as initiators of polyene cyclisations, giving rise to synthetically useful cyclic bromoalkenes.
Gold(I)-catalyzed cycloisomerisations of 1,n-enynes as well as the reactions of these substrates with many nucleophiles allow the construction of complex carbo- and heterocyclic compounds by the selective activation of the alkyne in the presence of many other functional groups.1 These transformations have been used as the key steps in the total synthesis of diverse natural products.2,3 In most cases, such as in substrates 1a,4 nucleophilic additions to 1,5-enynes proceed by an overall 6-endo-dig/endo-trig process leading to the formation of cyclohexenes 2avia a bicyclic gold(I) carbene intermediate.5,6 However, hydroxy-1,5-enyne 1b reacts in the presence of AuCl3 to exclusively form cyclopentene 2b by a 5-endo-dig/exo-dig cyclisation in which the alcohol adds to the alkene with an anti-Markovnikov regioselectivity (Scheme 1).4 Similar transformations have been reported with 1,6-enynes bearing hydroxyl7 or carboxylic acid groups at the alkenyl chain.8,9
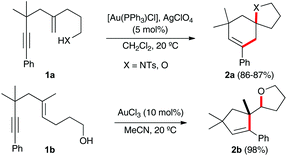 | ||
| Scheme 1 Gold(I)-catalysed intramolecular heterocyclisation of 1,5-enynes 1a–b.4 | ||
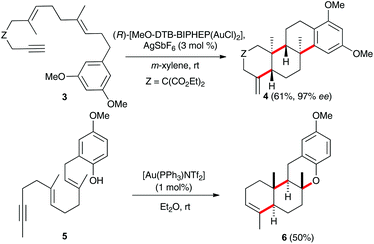
Remarkable examples of gold(I)-catalysed cyclisations in which up to 2–3 carbon–carbon bonds were formed had been reported by Toste,9 Michelet,10 and by other groups.11 Thus, the 6-exo-dig/endo-trig cyclisation of 3 with a chiral gold(I) catalyst leads to tetracyclic compound 4 in a highly enantioselective process (Scheme 2).9,12,13 Similar intriguing is the 6-endo-dig/endo-trig cyclisation of 5, which is terminated by trapping of the cationic intermediate by the phenol to form 6.10a Although highly ordered, concerted mechanisms have been proposed for these polycyclisations,8 step-wise processes have been suggested for reactions involving external nucleophiles and in other processes.1j,3d,11a,14
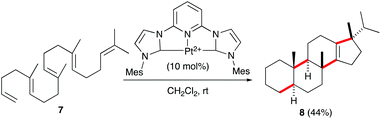
Recently, the group of Gagné reported the polycyclisation of pentanene 7 with a pincer-platinum(II) catalyst to give steroid-like product 8 (Scheme 3).15 This fascinating transformation, clearly reminiscent of sterol biosynthesis from squalene in bacteria,16 allows the formation of four C–C bonds in a single step.17
We decided to explore the possibility of performing gold(I)-catalysed cyclisations analogous to that of pentanene 7 but with a terminal alkyne instead of an alkene to form steroid-like products functionalised with an alkene at the A ring. As a first step towards this goal, we studied a set of 1,5-enynes substituted with different alcohols, phenols, arenes, and heteroarenes as potential nucleophiles. In addition to terminal alkynes, which were not broadly studied previously,10,18 we decided to employ 1-bromo-1,5-enynes as the initiators of cyclisation. Surprisingly, bromoalkynes have seldom been used in gold-catalysed cyclisations.19,20
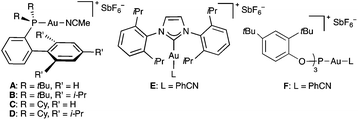
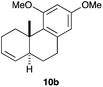
At the outset, we examined the cyclisation of (E)-2,6-dimethyldeca-1,5-dien-9-yne (9a) with gold(I) catalysts A–F bearing electronically different bulky groups (Table 1). In all cases, trans-fused hexahydronaphthalene 10a was cleanly obtained as the major product after 1 h by using just 1 mol% catalyst. As we have observed before in other contexts, the best yields were obtained with cationic gold(I) complexes bearing very bulky biphenylphosphine ligands (Buchwald ligands).1j,21 In this particular instance, cationic dicyclohexylphosphinobiphenyl gold(I) complex C outperforms Johnphos, t-BuXphos, and Xphos complexes A, B, and D (Table 1, entries 1–4).
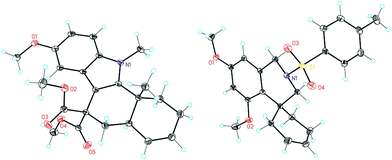
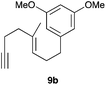
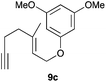
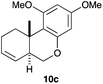
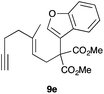
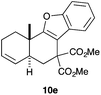
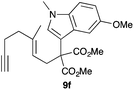
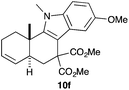
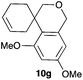
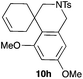
Complex C (3 mol%) was used as the catalyst in the cyclisation of aryl substituted 1,5-enynes (Table 2). The reaction of substrates 9b–f bearing electron-rich aromatic and heteroaromatic rings as cyclisation terminators proceeds to give products 10b–f as single diastereomers in good yields in all cases, with the exception of 10c, which was obtained in 54% yield (Table 2, entries 1–5). Similar results have been obtained with different metal catalysts using 1,5-enynes analogous to 9b–c with a methyl substituent at the terminal alkyne.10b,12b,13 The trans-relative configuration was confirmed by X-ray diffraction in the case of indole derivative 10f (Fig. 1), which has a carbon skeleton somewhat related to that of the alkaloids aristomakinine and aristomakine, although for these natural products a cis-hexalin structure has been assigned.22 The gold(I)-catalysed cyclisation of 1,5-enynes 9g–h substituted at C-5, gave spirocyclic derivatives 10g–h in good to excellent yields (Table 2, entries 6 and 7). The structure of 10h was confirmed by X-ray diffraction (Fig. 1).
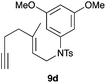
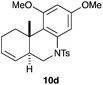
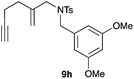
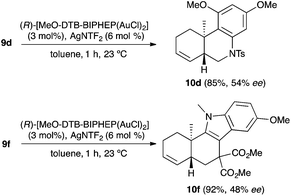
We also tested several chiral gold(I) catalysts in the cyclisation of enynes 9d and 9f.23,24 However, despite the excellent cyclisation yields, the enantioselectivities achieved with the dinuclear gold(I) complex of [MeO–DTB–BIPHEP] in the presence of AgNTf2 were only moderate (54 and 48% ee, respectively) (Scheme 4).25
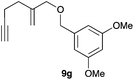
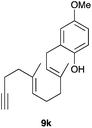
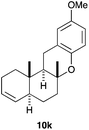
The intramolecular addition of alcohols and phenols was briefly studied with substrates 9i–k (Table 3). As expected considering the precedents,10 products 10i–k were obtained in good to excellent yields. Spirocyclisations similar to that of 9i to form 10i could be applied for the synthesis of analogues of the natural product filifolinol and other more complex, biologically active compounds with a spirobenzofuran structure.26
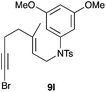
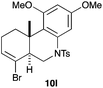
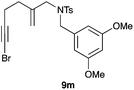
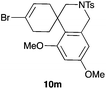
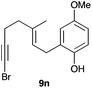
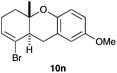
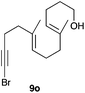
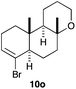
The cyclisation of 1-bromo-1,5-enynes 9l–n and 1-bromo-1,5,9-dienyne 9o with catalyst C took place uneventfully under the usual reaction conditions to give products 10l–o in good yields (Table 4). These results show that bromoalkynes are perfectly suitable initiators of gold(I)-catalysed polycyclisations. The final products are bromoalkenes, which could be further functionalized by metal-catalysed cross-couplings, carbonylations, or by other methods.
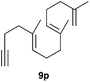
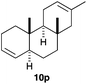
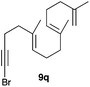
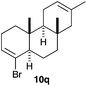
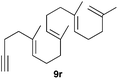
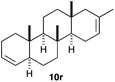
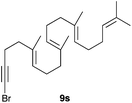
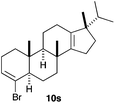
The polycyclisation of trienynes 9p–q and tetraenynes 9r–s was similarly performed with catalyst C (1–3 mol%) to give tri- and tetracyclic compounds 10p–s (Table 5). Considering that four C–C bonds are formed in a single step, the catalytic transformations of tetraenynes 9r–s into 10r–s are quite remarkable and comparable to that achieved by Gagné in the Pt(II)-catalysed polycyclisation of pentanene 7 (Scheme 3).15,27 However, in our case a lower catalyst loading is required and the final tetracyclic derivatives 10r–s feature two differently substituted double bonds. In 10s, the alkenyl bromide offers a handle for further functionalisation of the A-ring.
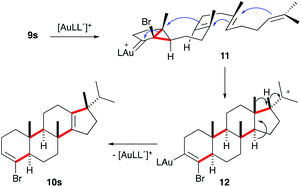
Presumably, the cyclisation of 9s to give 10s proceeds by the initial formation of gold(I)-carbene intermediate5,11 which triggers a cascade process to form secondary carbocation 12 (Scheme 5). The final tetracyclic compound 10s is then formed by Wagner–Meerwein 1,2 H and Me migrations,15,28 followed by the proton elimination and protonolysis of the alkenyl-gold(I) bond.
In summary, building upon previous studies,9,10 we have extended the polycyclisation of polyenynes up to the simultaneous formation of four C–C bonds. These reactions are performed under mild conditions with low catalyst loadings (1–3 mol%) of a cationic dicyclohexylphosphinobiphenyl gold(I)complex with a weakly coordinating acetonitrile ligand. In addition to terminal alkynes, we have also found that bromoalkynes can be used as the initiators of polyene cyclisations, leading to synthetically useful cyclic bromoalkenes. Further work on the development of broad scope and practical solutions of the asymmetric polycyclisation of polyeneynes is underway.
Acknowledgements
We acknowledge funding from MINECO/FEDER, UE (CTQ2016-75960-P), MINECO-Severo Ochoa Excellence Accreditation 2014–2018, (SEV-2013-0319), the European Research Council (Advanced Grant No. 321066), the AGAUR (2014 SGR 818), and CERCA Programme/Generalitat de Catalunya. We also thank the ICIQ X-ray diffraction unit and CELLEX-ICIQ HTE laboratory.References
- Selected reviews: (a) L. Zhang, J. Sun and S. A. Kozmin, Adv. Synth. Catal., 2006, 348, 2271–2296 CrossRef CAS; (b) A. Fürstner and P. W. Davies, Angew. Chem., Int. Ed., 2007, 46, 3410–3449 CrossRef PubMed; (c) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180–3211 CrossRef CAS PubMed; (d) E. Jiménez-Núñez and A. M. Echavarren, Chem. Rev., 2008, 108, 3326–3350 CrossRef PubMed; (e) D. J. Gorin, B. D. Sherry and F. D. Toste, Chem. Rev., 2008, 108, 3351–3378 CrossRef CAS PubMed; (f) V. Michelet, P. Y. Toullec and J.-P. Genêt, Angew. Chem., Int. Ed., 2008, 47, 4268–4315 CrossRef CAS PubMed; (g) A. Fürstner, Chem. Soc. Rev., 2009, 38, 3208–3221 RSC; (h) C. Aubert, L. Fensterbank, P. Garcia, M. Malacria and A. Simonneau, Chem. Rev., 2011, 111, 1954–1993 CrossRef CAS PubMed; (i) N. Krause and C. Winter, Chem. Rev., 2011, 111, 1994–2009 CrossRef CAS PubMed; (j) C. Obradors and A. M. Echavarren, Acc. Chem. Res., 2014, 47, 902–912 CrossRef CAS PubMed; (k) L. Fensterbank and M. Malacria, Acc. Chem. Res., 2014, 47, 953–965 CrossRef CAS PubMed; (l) R. Dorel and A. M. Echavarren, Chem. Rev., 2015, 115, 9028–9072 CrossRef CAS PubMed.
- Selected reviews: (a) A. S. K. Hashmi and M. Rudolph, Chem. Soc. Rev., 2008, 37, 1766–1775 RSC; (b) M. Rudolph and A. S. K. Hashmi, Chem. Soc. Rev., 2012, 41, 2448–2462 RSC; (c) A. Fürstner, Acc. Chem. Res., 2014, 47, 925–938 CrossRef PubMed; (d) Y. Zhang, T. Luo and Z. Yang, Nat. Prod. Rep., 2014, 31, 489–503 RSC.
- Work towards the synthesis of natural products by using gold catalysis from our group: (a) E. Jiménez-Núñez, K. Molawi and A. M. Echavarren, Chem. Commun., 2009, 7327–7329 RSC; (b) K. Molawi, N. Delpont and A. M. Echavarren, Angew. Chem., Int. Ed., 2010, 49, 3517–3519 CrossRef CAS PubMed; (c) M. Gaydou, R. E. Miller, N. Delpont, J. Ceccon and A. M. Echavarren, Angew. Chem., Int. Ed., 2013, 52, 6396–6399 CrossRef CAS PubMed; (d) J. Carreras, M. Livendahl, P. R. McGonigal and A. M. Echavarren, Angew. Chem., Int. Ed., 2014, 53, 4896–4899 CrossRef CAS PubMed; (e) A. Homs, M. E. Muratore and A. M. Echavarren, Org. Lett., 2015, 17, 461–463 CrossRef CAS PubMed; (f) B. Ranieri, C. Obradors, M. Mato and A. M. Echavarren, Org. Lett., 2016, 18, 1614–1617 CrossRef CAS PubMed; (g) M. S. Kirillova, M. E. Muratore, R. Dorel and A. M. Echavarren, J. Am. Chem. Soc., 2016, 138, 3671–3674 CrossRef CAS PubMed; (h) J. Carreras, M. S. Kirillova and A. M. Echavarren, Angew. Chem., Int. Ed., 2016, 55, 7121–7125 CrossRef CAS PubMed; (i) R. Dorel and A. M. Echavarren, J. Org. Chem., 2016, 81, 8444–8454 CrossRef CAS PubMed.
- L. Zhang and S. A. Kozmin, J. Am. Chem. Soc., 2005, 127, 6962–6963 CrossRef CAS PubMed.
- M. R. Luzung, J. P. Markham and F. D. Toste, J. Am. Chem. Soc., 2004, 126, 10858–10859 CrossRef CAS PubMed.
- V. López-Carrillo, N. Huguet, Á. Mosquera, A. M. Echavarren and A. M, Chem. – Eur. J., 2011, 17, 10972–10978 CrossRef.
- C. Nieto-Oberhuber, M. P. Muñoz, S. López, E. Jiménez-Núñez, C. Nevado, E. Herrero-Gómez, M. Raducan and A. M. Echavarren, Chem. – Eur. J., 2006, 12, 1677–1693 CrossRef CAS PubMed . Corrigendum: Chem. – Eur. J., 2008, 14, 5096.
- A. Fürstner and L. Morency, Angew. Chem., Int. Ed., 2008, 47, 5030–5033 CrossRef PubMed.
- S. G. Sethofer, T. Mayer and F. D. Toste, J. Am. Chem. Soc., 2010, 132, 8276–8277 CrossRef CAS PubMed.
- (a) P. Y. Toullec, T. Blarre and V. Michelet, Org. Lett., 2009, 11, 2888–2891 CrossRef CAS PubMed; (b) A. Pradal, Q. Chen, P. F. dit Bel, P. Y. Toullec and V. Michelet, Synlett, 2012, 74–79 CAS.
- (a) S. Böhringer and F. Gagosz, Adv. Synth. Catal., 2008, 350, 2617–2630 CrossRef; (b) A. Buzas, F. Istrate, X. F. Le Goff, Y. Odabachian and F. Gagosz, J. Organomet. Chem., 2009, 694, 515–519 CrossRef CAS; (c) Y. Lee, C. Lim, S. Kim and S. Shin, Bull. Korean Chem. Soc., 2010, 31, 670–677 CrossRef CAS; (d) P.-J. Cai, Y. Wang, C.-H. Liu and Z.-X. Yu, Org. Lett., 2014, 16, 5898–5901 CrossRef CAS PubMed; (e) A. Danda, K. Kumar and H. Waldmann, Chem. Commun., 2015, 51, 7536–7539 RSC; (f) R. Wildermuth, K. Speck and T. Magauer, Synthesis, 2016, 1814–1824 CAS.
- Related indium(III)-catalysed cyclisations: (a) K. Surendra, W. Qiu and E. J. Corey, J. Am. Chem. Soc., 2011, 133, 9724–9726 CrossRef CAS PubMed; (b) W.-W. Qiu, K. Surendra, L. Yin and E. J. Corey, Org. Lett., 2011, 13, 5893–5895 CrossRef CAS PubMed; (c) K. Surendra and E. J. Corey, J. Am. Chem. Soc., 2014, 136, 10918–10920 CrossRef CAS PubMed.
- Related mercury(II)-catalysed cyclisations: H. Imagawa, T. Iyenaga and M. Nishizawa, Org. Lett., 2005, 7, 451–453 CrossRef CAS PubMed.
- (a) N. Huguet and A. M. Echavarren, Synlett, 2012, 49–53 CAS; (b) P. Calleja, M. E. Muratore, T. Jiménez and A. M. Echavarren, Synthesis, 2016, 3183–3198 CAS; (c) P. Calleja, Ó. Pablo, B. Ranieri, M. Gaydou, A. Pitaval, M. Moreno, M. Raducan and A. M. Echavarren, Chem. – Eur. J., 2016, 22, 13613–13618 CrossRef CAS PubMed.
- (a) M. J. Geier and M. R. Gagné, J. Am. Chem. Soc., 2014, 136, 3032–3035 CrossRef CAS PubMed; (b) R. J. Felix, C. Munro-Leighton and M. R. Gagné, Acc. Chem. Res., 2014, 47, 2319–2331 CrossRef CAS PubMed.
- (a) R. A. Yoder and J. N. Johnston, Chem. Rev., 2005, 105, 4730–4756 CrossRef CAS PubMed; (b) D. J. Tantillo, Chem. Soc. Rev., 2010, 39, 2847–2854 RSC; (c) Y. Gao, R. B. Honzatko and R. J. Petters, Nat. Prod. Rep., 2012, 29, 1153–1175 RSC.
- Recent leading references on enantioselective polyene cyclizations: (a) C. N. Ungarean, E. H. Southgate and D. Sarlah, Org. Biomol. Chem., 2016, 14, 5454–5467 RSC; (b) R. C. Samanta and H. Yamamoto, J. Am. Chem. Soc., 2017, 139, 1460–1463 CrossRef CAS PubMed.
- Only two of the substrates reported ref. 10a and b that underwent cyclisation were terminal alkynes.
- (a) T. Matsuda, S. Kadowaki, Y. Yamaguchi and M. Murakami, Chem. Commun., 2008, 2744–2746 RSC; (b) F. Barabé, G. Bétournay, G. Bellavance and L. Barriault, Org. Lett., 2009, 11, 4236–4238 CrossRef PubMed; (c) K. Speck, K. Karaghiosoff and T. Magauer, Org. Lett., 2015, 17, 1982–1985 CrossRef CAS PubMed; (d) Intramolecular hydroarylation of bromoalkynes: V. Mamane, P. Hannen and A. Fürstner, Chem. – Eur. J., 2004, 10, 4556–4575 CrossRef CAS PubMed.
- Cyclisation of a 1-bromo-1,5-enyne promoted by N-iodosuccinimide: B. Crone, S. F. Kirsch and K.-D. Umland, Angew. Chem., Int. Ed., 2010, 49, 4661–4664 CrossRef CAS PubMed.
- (a) B. Ranieri, I. Escofet and A. M. Echavarren, Org. Biomol. Chem., 2015, 13, 7103–7118 RSC; (b) R. Miller, J. Carreras, M. E. Muratore, M. Gaydou, F. Camponovo and A. M. Echavarren, J. Org. Chem., 2016, 81, 1839–1849 CrossRef CAS PubMed.
- S. Burkard and H.-J. Borsehberg, Helv. Chim. Acta, 1990, 73, 298–302 CrossRef CAS.
- Recent reviews on enantioselective gold(I)-catalysed reactions: (a) S. Sengupta and X. Shi, ChemCatChem, 2010, 2, 609–619 CrossRef CAS; (b) A. Pradal, P. Y. Toullec and V. Michelet, Synthesis, 2011, 1501–1514 CAS; (c) W. Zi and F. D. Toste, Chem. Soc. Rev., 2016, 45, 4567–4589 RSC; (d) Y. Li, W. Li and J. Zhang, Chem. – Eur. J., 2017, 23, 467–512 CrossRef CAS PubMed.
- Recent lead references on enantioselective gold(I)-catalysed reactions: (a) N. Delpont, I. Escofet, P. Pérez-Galán, D. Spiegl, M. Raducan, C. Bour, R. Sinisi and A. M. Echavarren, Catal. Sci. Technol., 2013, 3, 3007–3012 RSC; (b) P. Aillard, D. Dova, V. Magné, P. Retailleau, S. Cauteruccio, E. Licandro, A. Voituriez and A. Marinetti, Chem. Commun., 2016, 52, 10984–10987 RSC; (c) Z. Wu, K. Isaac, P. Retailleau, J.-F. Betzer, A. Voituriez and A. Marinetti, Chem. – Eur. J., 2016, 22, 3278–3281 CrossRef CAS PubMed; (d) E. González-Fernández, L. D. M. Nicholls, L. D. Schaaf, C. Farès, C. W. Lehmann and M. Alcarazo, J. Am. Chem. Soc., 2017, 139, 1428–1431 CrossRef PubMed.
- The absolute configurations of the major enantiomer of 10d and 10f were tentatively assigned following that reported by Toste in the cyclization of substrate 3 with the same dinuclear gold(I) precatalyst.8.
- (a) B. J. Bradbury, P. Bartyzel, T. S. Kaufman, M. J. Nieto, R. D. Sindelar, S. M. Scesney, B. R. Gaumond and H. C. Marsh, J. Med. Chem., 2003, 46, 2697–2705 CrossRef CAS PubMed; (b) E. L. Larghi, M. A. Operto, R. Torres and T. S. Kaufman, Eur. J. Med. Chem., 2012, 55, 74–84 CrossRef CAS PubMed.
- Cu-Mediated oxidation in Pt(II)-catalysed cyclisations lead to alcohols and ketones: M. J. Geier and M. R. Gagné, Organometallics, 2013, 32, 380–383 CrossRef CAS PubMed.
- J. G. Sokol, C. S. Korapala, P. S. White, J. J. Becker and M. R. Gagné, Angew. Chem., Int. Ed., 2011, 50, 5658–5661 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1530340 and 1530341. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ob00235a |
| This journal is © The Royal Society of Chemistry 2017 |