Abstract
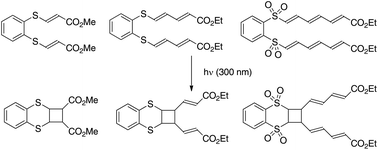
This study focusses on the [2 + 2]-photocycloaddition of a symmetric polyenic system tethered by an aryl bis-sulfide or sulfone platform. Using direct irradiation or photosensitization, no expected ladderane product was isolated. In most cases, only tricyclic products including a single cyclobutane moiety were formed. Irradiation of bis-acrylic precursors in water with encapsulation by a host (cyclodextrin or cucurbituril) provided a stereoselective access to valuable cyclobutyl adducts.
- This article is part of the themed collection: Polycyclic Reactions in Synthesis and Biosynthesis


 Please wait while we load your content...
Please wait while we load your content...