Themed collection Understanding biosynthetic protein-protein interactions

Front cover

Front cover

Inside front cover

Understanding biosynthetic protein–protein interactions
The Natural Product Reports themed issue on Understanding biosynthetic protein–protein interactions is introduced by the Guest Editors, David Ackerley, Gregory Challis and Max Cryle.

Nat. Prod. Rep., 2018,35, 1118-1119
https://doi.org/10.1039/C8NP90037J
The role of protein–protein interactions in the biosynthesis of ribosomally synthesized and post-translationally modified peptides
This review covers the role of protein–protein complexes in the biosynthesis of selected ribosomally synthesized and post-translationally modified peptide (RiPP) classes.
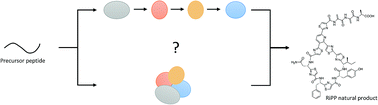
Nat. Prod. Rep., 2019,36, 1576-1588
https://doi.org/10.1039/C8NP00064F
Dynamic metabolic solutions to the sessile life style of plants
Plants are sessile organisms. To compensate for not being able to escape when challenged by unfavorable growth conditions, pests or herbivores, plants have perfected their metabolic plasticity by having developed the capacity for on demand dynamic biosynthesis and storage of a plethora of phytochemicals.
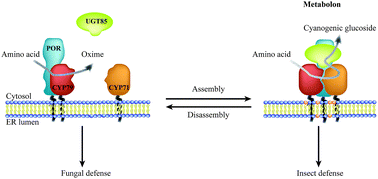
Nat. Prod. Rep., 2018,35, 1140-1155
https://doi.org/10.1039/C8NP00037A
The many faces and important roles of protein–protein interactions during non-ribosomal peptide synthesis
Non-ribosomal peptide synthetase (NRPS) machineries are complex, multi-domain proteins that are responsible for the biosynthesis of many important, peptide-derived compounds. In this review, we present the current state of understanding of the protein–protein interactions that govern NRPS-mediated biosynthesis.
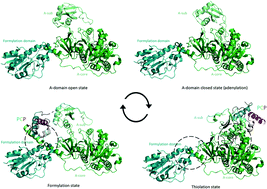
Nat. Prod. Rep., 2018,35, 1120-1139
https://doi.org/10.1039/C8NP00038G
Protein–protein interactions in polyketide synthase–nonribosomal peptide synthetase hybrid assembly lines
The protein–protein interactions in polyketide synthase–nonribosomal peptide synthetase hybrids are summarized and discussed.
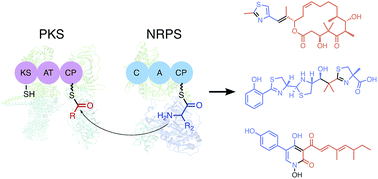
Nat. Prod. Rep., 2018,35, 1185-1209
https://doi.org/10.1039/C8NP00022K
Structural, functional and evolutionary perspectives on effective re-engineering of non-ribosomal peptide synthetase assembly lines
Salutary lessons from recent progress in re-engineering non-ribosomal peptide synthetase assembly lines, emphasizing effective strategies and key protein–protein interactions.
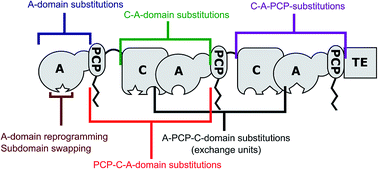
Nat. Prod. Rep., 2018,35, 1210-1228
https://doi.org/10.1039/C8NP00036K
Trapping interactions between catalytic domains and carrier proteins of modular biosynthetic enzymes with chemical probes
A review of chemical probes used to characterize interactions between carrier and catalytic domains of modular NRPS and PKS enzymes.
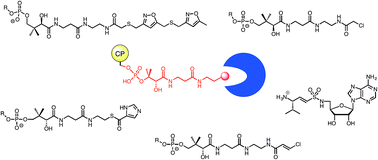
Nat. Prod. Rep., 2018,35, 1156-1184
https://doi.org/10.1039/C8NP00044A
Protein–protein interactions in trans-AT polyketide synthases
An extensive and highly programmed set of inter- and intra-subunit protein–protein interactions controls chain assembly by trans-AT polyketide synthases.
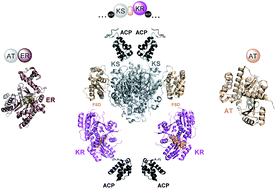
Nat. Prod. Rep., 2018,35, 1097-1109
https://doi.org/10.1039/C8NP00066B
Protein–protein interactions in “cis-AT” polyketide synthases
Protein–protein interactions of cis-AT polyketide synthases are dominated by the travels of the ACP domain to the active site entrance of each catalytic domain.
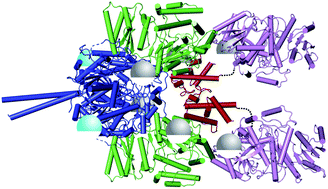
Nat. Prod. Rep., 2018,35, 1082-1096
https://doi.org/10.1039/C8NP00058A
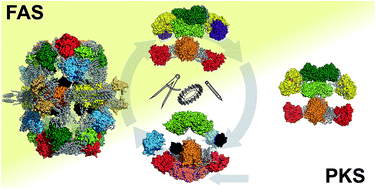
The architectures of iterative type I PKS and FAS
The architectures of fatty acid synthases and iterative polyketide synthases are remarkably divergent despite their related biosynthetic logics.
Nat. Prod. Rep., 2018,35, 1046-1069
https://doi.org/10.1039/C8NP00039E
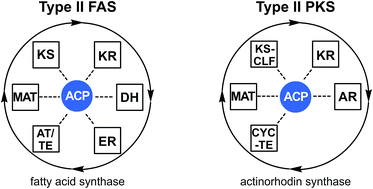
Type II fatty acid and polyketide synthases: deciphering protein–protein and protein–substrate interactions
Metabolites from type II fatty acid synthase (FAS) and polyketide synthase (PKS) pathways differ broadly in their identities and functional roles.
Nat. Prod. Rep., 2018,35, 1029-1045
https://doi.org/10.1039/C8NP00040A
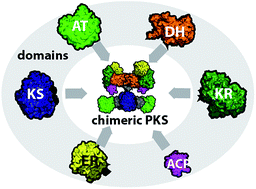
Engineering strategies for rational polyketide synthase design
In this review, we highlight strategies in engineering polyketide synthases (PKSs). We focus on important protein–protein interactions that constitute an intact PKS assembly line.
Nat. Prod. Rep., 2018,35, 1070-1081
https://doi.org/10.1039/C8NP00030A
About this collection
This themed issue is Guest Edited by Professor David Ackerley (Victoria University), Professor Greg Challis and Professor Max Cryle (Monash University).
Specialised metabolism is dependent on complex biosynthetic pathways that often employ enzymes with multiple catalytic domains, requiring precisely coordinated interactions. Recent structural and biochemical studies on these systems have led to rapid advances in our understanding of how protein-protein interactions are not only crucial to the core functions of such enzymes, but how they regulate and enable the assembly of complex natural products.
This themed issue focuses on the roles and the importance of protein–protein interactions, particularly with reference to specialised metabolite megaenzyme synth(et)ases, but also to pathways involving other types of biosynthetic enzymes, and the implications that this has for function as well as redesign of complex biosynthetic pathways.