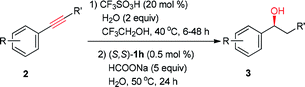Open Access Article
Open Access ArticleOne-pot synthesis of chiral alcohols from alkynes by CF3SO3H/ruthenium tandem catalysis†
Huan
Liu
,
Sensheng
Liu
,
Haifeng
Zhou
 *,
Qixing
Liu
and
Chunqin
Wang
*,
Qixing
Liu
and
Chunqin
Wang
Research Center of Green Pharmaceutical Technology and Process, Hubei Key Laboratory of Natural Products Research and Development, College of Biological and Pharmaceutical Sciences, China Three Gorges University, Yichang 443002, China. E-mail: zhouhf@ctgu.edu.cn
First published on 19th April 2018
Abstract
A practical one-pot synthesis of chiral alcohols from readily available alkynes via tandem catalysis by the combination of CF3SO3H and a fluorinated chiral diamine Ru(II) complex in aqueous CF3CH2OH is described. Very interestingly, the combination of fluorinated catalysts and solvent exhibits a positive fluorine effect on the reactivity and enantioselectivity. A range of chiral alcohols with wide functional group tolerance was obtained in high yield and excellent stereoselectivity under simple and mild conditions.
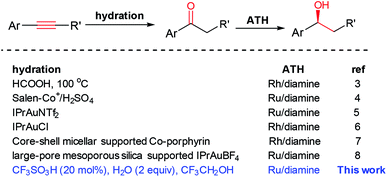
Pot-economic reactions as alternatives to traditional multistep synthetic procedures have received great attention. The significant benefits of a one-pot reaction include simple starting materials and minimum isolation/purification processes.1 The direct conversion of alkynes into alcohols is of great importance because of the readily available alkynes as well as the valuable alcohols. However, the one-pot conversion of alkynes into alcohols through a tandem hydration/reduction process is still challengeable because of the conflict of extrinsic reaction conditions and the incompatible nature of the catalysts.2 The first one-pot asymmetric synthesis of chiral alcohols from alkynes via hydration/asymmetric transfer hydrogenation (ATH) was reported by Xiao's group. In their process, formic acid was used as a solvent for the hydration step at 100 °C, and then the ATH was conducted at neutral conditions by adding a large amount of NaOH (Scheme 1).3 After that, Sun and co-workers described the same conversion catalyzed by the combination of salen–Co3+ and a chiral ruthenium complex with the aid of H2SO4.4 However, it is not applicable for electron-deficient alkynes. Subsequently, the similar transformation with homogeneous bimetallic catalysts like Au–Ru5 and Au–Rh6 has also been realized. In addition, the heterogeneous bimetallic catalytic systems like core–shell micellar supported Co/porphyrin and chiral rhodium/diamine complexes,7 large-pore mesoporous silica supported Au/carbene and chiral ruthenium/diamine dual complexes8 were also applied in this transformation. Despite these achievements, it is highly desirable to develop simple, efficient, and compatible catalytic systems under mild conditions for the conversion of alkynes into chiral alcohols from both a practical standpoint and an environmental point of view.
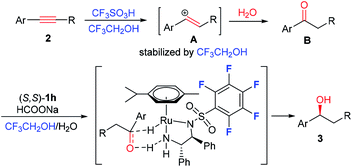
In 2016, a Markovnikov-type alkyne hydration under simple conditions, using 20 mol% trifluoromethanesulfonic acid (CF3SO3H) as the catalyst and 2,2,2-trifluoroethanol (CF3CH2OH) as the solvent was developed by Li and coworkers.9 It was reported the CF3SO3− could be counter anion of chiral Ru/diamine complexes, which were used as privileged catalysts for the AH or ATH of ketones.10,11
We reason that the conversion of alkynes into chiral alcohols will be realized by tandem catalysis with CF3SO3H and chiral Ru/diamine complexes as the catalysts. Most importantly, it not only makes the catalytic systems simple, but also cleverly avoids the incompatible problem of different catalysts in one-pot reaction. To continue our interest in asymmetric synthesis of chiral alcohols,12 we describe an easy operation process for a pot-economic synthesis of chiral alcohols from readily available alkynes via tandem catalysis under simple conditions, using CF3SO3H catalyzed hydration of alkynes in CF3CH2OH, followed by ATH with fluorinated chiral diamine Ru(II) complex as the catalyst and sodium formate as the hydrogen source.
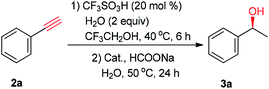
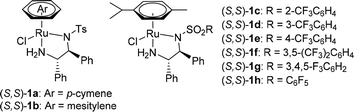
Initially, the hydration of phenylacetylene (2a) was carried out with 20 mol% CF3SO3H as the catalyst and 2 mL of CF3CH2OH as the solvent in the presence of 2 equiv. of H2O.9 As monitored by gas chromatography (GC), 2a was converted to acetophenone quantitatively at 40 °C for 6 h.13 Then, 0.5 mol% (S,S)-1a, 5 equiv. of HCOONa, and 2 mL of H2O were added. The subsequent ATH was conducted at 50 °C for 24 h, affording the desired (S)-1-phenylethanol (3a) in 41% yield and 93% ee (Table 1, entry 1). Next, the chiral ruthenium/diamine complexes including 1b–1h were screened. Interestingly, it was found that a fluorine effect on the reactivity and enantioselectivity was observed in this tandem reaction.14 For example, the fluorinated chiral diamine Ru(II) complexes 1c–1h exhibit high reactivity and excellent enantioselectivity (entries 3–8). Among which, the best yield and ee value were provided by the chiral N-pentafluorobenzenesulfonyl-1,2-diphenylethylenediamine Ru(II) complex (S,S)-1h, giving 3a in 95% yield and 97% ee (entry 8). Then, other fluorinated alcohols like hexafluoro-2-propanol (HFIP) could also give the desired product in 95% ee but with only 24% yield (entry 9). Finally, other hydrogen sources were also examined. For example, no desired product was detected using an acidic HCOOH–NEt3 azeotrope (molar ratio F/T = 5/2) as hydrogen source (entry 10). By contrast, 3a was obtained in 46% yield and 95% ee using slightly basic HCOOH–NEt3 mixture (molar ratio F/T = 1.1/1) as hydrogen source (entry 11).15 Based on above results, the optimal reaction conditions were set as follow: 20 mol% CF3SO3H coupled with 0.5 mol% (S,S)-1h as the catalysts, 5 equiv. of HCOONa as the hydrogen source, and CF3CH2OH/H2O (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent (entry 8).
1) as the solvent (entry 8).
| Entry | Cat. | Hydrogen source | Yield (%) | ee (%) |
|---|---|---|---|---|
| a Reaction conditions: phenylacetylene (2a; 5 mmol), CF3SO3H (20 mol%), H2O (2 equiv.), CF3CH2OH (2 mL), 40 °C, 6 h; then 0.5 mol% catalyst, hydrogen source (5 equiv.), and H2O (2 mL) were added, 50 °C, 24 h. The yield was determined by GC with an internal standard (mesitylene). The ee values were determined by HPLC analysis. b Hexafluoro-2-propanol (HFIP) was used as a solvent. c 0.5 mL of HCOOH/NEt3 mixture was used, the data in the brackets are molar ratio. | ||||
| 1 | (S,S)-1a | HCOONa (5 equiv.) | 41 | 93 |
| 2 | (S,S)-1b | HCOONa (5 equiv.) | <5 | — |
| 3 | (S,S)-1c | HCOONa (5 equiv.) | 80 | 87 |
| 4 | (S,S)-1d | HCOONa (5 equiv.) | 83 | 96 |
| 5 | (S,S)-1e | HCOONa (5 equiv.) | 78 | 79 |
| 6 | (S,S)-1f | HCOONa (5 equiv.) | 79 | 94 |
| 7 | (S,S)-1g | HCOONa (5 equiv.) | 84 | 94 |
| 8 | (S,S)-1h | HCOONa (5 equiv.) | 95 | 97 |
| 9b | (S,S)-1h | HCOONa (5 equiv.) | 24 | 95 |
| 10c | (S,S)-1h | HCOOH/NEt3 (5:2) | 0 | — |
| 11c | (S,S)-1h | HCOOH/NEt3 (1.1:1) | 46 | 95 |
|
||||
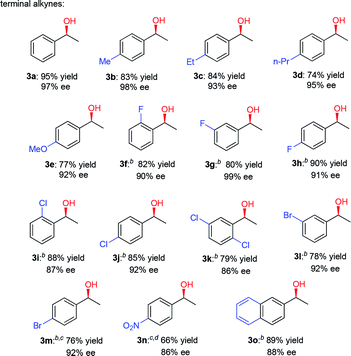
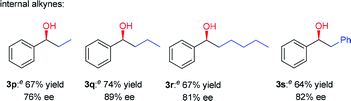
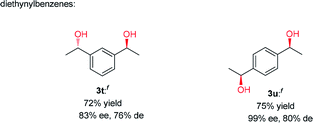
Having established a compatible catalytic system for the one-pot conversion of alkynes into chiral alcohols, the scope of various alkynes was then investigated. As summarized in Table 2, the internal aromatic alkynes were examined first. For examples, the reaction of phenylacetylene derivatives 2b–2e bearing electron-donating groups (Me, Et, n-Pr, MeO) performed smoothly under the optimized conditions, giving the corresponding chiral alcohols 3b–3e in 74–95% yield and 92–98% ee. By contrast, in the case of electron-deficient phenylacetylene derivatives 2f–2n bearing electron-withdrawing groups (F, Cl, Br, NO2), the hydration step requires higher temperature and longer time. Due to poor solubility of 4-bromophenylacetylene (2m) and 4-nitrophenylacetylene (2n) in CF3CH2OH, the HFIP was used instead. In addition to phenylacetylene derivatives, the 2-ethynylnaphthalene (2o) could also be converted to alcohol 3o in 89% yield and 88% ee successfully. Next, the internal aromatic alkynes 2p–2s were also subjected to this tandem reaction, and the corresponding alcohols 3p–3s were isolated with 64–74% yield and 76–89% ee. Most importantly, the direct conversion of 1,3-diethynylbenzene (2t) and 1,4-diethynylbenzene (2u) into chiral diols 3t and 3u was achieved, with high yield, good enantiomeric excess (ee) and diastereomeric excess (de). Finally, the alkynes like 3-ethynylpyridine (2v), 2-ethynylthiophene (2w), 3-phenylpropargyl alcohol (2x), and methyl phenylpropiolate (2y) were also attempted, but no desired product was obtained with this catalytic system.
| a Reaction conditions: alkyne (5 mmol), CF3SO3H (20 mol%), H2O (2 equiv.), CF3CH2OH (2 mL), 40 °C, 6 h, then add 0.5 mol% (S,S)-1h, HCOONa (5 equiv.), H2O (2 mL), 50 °C, 24 h, isolated yield, the ee values were determined by HPLC analysis. b Conditions were 70 °C and 12 h for hydration step. c HFIP was used as a solvent. d Conditions were 70 °C and 48 h for hydration step. e Conditions were 40 °C and 48 h for hydration step. f CF3SO3H (40 mol%), H2O (4 equiv.), CF3CH2OH (2 mL), 70 °C, 48 h, then add 1 mol% (S,S)-1h, HCOONa (10 equiv.), H2O (2 mL), 50 °C, 48 h. |
|---|
|
|
|
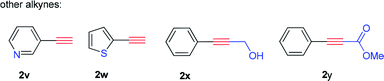
|
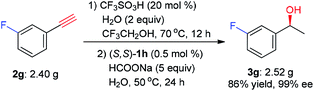
To further demonstrate the potential application of this one-pot tandem process, a gram scale reaction with 3-fluorophenylacetylene (2g) as the substrate was conducted. As shown in Scheme 2, the chiral alcohol 3g was obtained in 86% yield and 99% ee, which demonstrates its suitability for large-scale reaction.
Very interestingly, the reactivity and enantioselectivity of this asymmetric tandem reaction could be enhanced by fluorinated catalysts and solvents (Table 1). As shown in Scheme 3, in the hydration step, the intermediate vinyl carbocation A could be stabilized by the fluorinated solvent (CF3CH2OH), which makes the hydration reaction proceed smoothly under mild conditions. By contrast, no reaction occurs if CF3CH2OH replaced by C2H5OH.9 In the ATH step, the (S,S)-1h containing fluorinated aryl moiety gives better yield and ee value, it may be ascribe to the positive fluorine effect between the fluorinated solvent and catalyst.16
In summary, we have developed a simple and efficient compatible catalytic system, using a fluorine-containing BrØnsted acid (CF3SO3H) coupled with a fluorinated chiral diamine Ru(II) complex as catalysts, and fluorinated alcohol (CF3CH2OH) as a solvent, which exhibits positive fluorine effect on the reactivity and enantioselectivity for the conversion of alkynes into chiral alcohols. Furthermore, the gram-scale reaction demonstrates its potential application.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support by the grants from the National Natural Science Foundation of China (21202092) and China Three Gorges University (KJ2014H008, KJ2014B084).Notes and references
- (a) Y. Hayashi, Chem. Sci., 2016, 7, 866–880 RSC; (b) D. B. Ramachary and S. Jain, Org. Biomol. Chem., 2011, 9, 1277–1300 RSC; (c) R. C. Simon, N. Richter, E. Busto and W. Kroutil, ACS Catal., 2014, 4, 129–143 CrossRef CAS; (d) M. J. Climent, A. Corma and S. Iborra, Chem. Rev., 2011, 111, 1072–1133 CrossRef CAS.
- (a) L. Li and S. B. Herzon, J. Am. Chem. Soc., 2012, 134, 17376–17379 CrossRef CAS; (b) J. L. Huang, F. Zhang and H. X. Li, Appl. Catal., A, 2012, 431–432, 95–103 CrossRef CAS.
- J. Li, C. Wang, D. Xue, Y. Wei and J. Xiao, Green Chem., 2013, 15, 2685–2689 RSC.
- S. Wang, C. Miao, W. Wang, Z. Lei and W. Su, ChemCatChem, 2014, 6, 1612–1616 CrossRef CAS.
- Q. Ye, T. Cheng, Y. Zhao, J. Zhao, R. Jin and G. Liu, ChemCatChem, 2015, 7, 1801–1805 CrossRef CAS.
- F. Li, N. Wang, L. Lu and G. Zhu, J. Org. Chem., 2015, 80, 3538–3546 CrossRef CAS.
- X. Xia, J. Meng, H. Wu, T. Cheng and G. Liu, Chem. Commun., 2017, 53, 1638–1641 RSC.
- J. Lu, J. Dimroth and M. Weck, J. Am. Chem. Soc., 2015, 137, 12984–12989 CrossRef CAS.
- W. Liu, H. Wang and C.-J. Li, Org. Lett., 2016, 18, 2184–2187 CrossRef CAS.
- ATH of ketones, selected examples: (a) S. Hashiguchi, A. Fujii, J. Takehara, T. Ikariya and R. Noyori, J. Am. Chem. Soc., 1995, 117, 7562–7563 CrossRef CAS; (b) J. Hannedouche, G. J. Clarkson and M. Wills, J. Am. Chem. Soc., 2004, 126, 986–987 CrossRef CAS; (c) A. M. Hayes, D. J. Morris, G. J. Clarkson and M. Wills, J. Am. Chem. Soc., 2005, 127, 7318–7319 CrossRef CAS; (d) D. S. Matharu, D. J. Morris, A. M. Kawamoto, G. J. Clarkson and M. Wills, Org. Lett., 2005, 7, 5489–5491 CrossRef CAS; (e) T. Ohkuma, N. Utsumi, K. Tsutsumi, K. Murata, C. Sandoval and R. Noyori, J. Am. Chem. Soc., 2006, 128, 8724–8725 CrossRef CAS; (f) F. K. Cheung, C. Lin, F. Minissi, A. L. Criville, M. A. Graham, D. J. Fox and M. Wills, Org. Lett., 2007, 9, 4659–4662 CrossRef CAS; (g) T. Touge, T. Hakamata, H. Nara, T. Kobayashi, N. Sayo, T. Saito, Y. Kayaki and T. Ikariya, J. Am. Chem. Soc., 2011, 133, 14960–14963 CrossRef CAS; (h) T. Touge, H. Nara, M. Fujiwhara, Y. Kayaki and T. Ikariya, J. Am. Chem. Soc., 2016, 138, 10084–10087 CrossRef CAS PubMed.
- AH of ketones, selected examples: (a) T. Ohkuma, K. Tsutsumi, N. Utsumi, N. Arai, R. Noyori and K. Murata, Org. Lett., 2007, 9, 255–257 CrossRef CAS; (b) T. Ohkuma, N. Utsumi, M. Watanabe, K. Tsutsumi, N. Arai and K. Murata, Org. Lett., 2007, 9, 2565–2567 CrossRef CAS; (c) N. Arai, H. Satoh, N. Utsumi, K. Murata, K. Tsutsumi and T. Ohkuma, Org. Lett., 2013, 15, 3030–3033 CrossRef CAS.
- (a) B. Wang, H. Zhou, G. Lu, Q. Liu and X. Jiang, Org. Lett., 2017, 19, 2094–2097 CrossRef CAS; (b) Q. Liu, C. Wang, H. Zhou, B. Wang, J. Lv, L. Cao and Y. Fu, Org. Lett., 2018, 20, 971–974 CrossRef CAS; (c) S. Liu, H. Liu, H. Zhou, Q. Liu and J. Lv, Org. Lett., 2018, 20, 1110–1113 CrossRef CAS.
- The reaction conditions were improved by enhancing the temperature and shortening the time compared to the reported conditions in ref. 9.
- The reactivity and stereoselectivity in asymmetric synthesis could be enhanced using fluorinated catalysts and solvents, selected examples: (a) T. Sugiishi, M. Matsugi, H. Hamamoto and H. Amii, RSC Adv., 2015, 5, 17269–17282 RSC; (b) H. Abe, H. Amii and K. Uneyama, Org. Lett., 2001, 3, 313–315 CrossRef CAS; (c) M.-W. Chen, Y. Duan, Q.-A. Chen, D.-S. Wang, C.-B. Yu and Y.-G. Zhou, Org. Lett., 2010, 12, 5075–5077 CrossRef CAS; (d) T. Touge and T. Arai, J. Am. Chem. Soc., 2016, 138, 11299–11305 CrossRef CAS; (e) Z. Yang, F. Chen, Y. He, N. Yang and Q.-H. Fan, Angew. Chem., Int. Ed., 2016, 55, 13863–13866 CrossRef CAS.
- X. Wu, X. Li, F. King and J. Xiao, Angew. Chem., Int. Ed., 2005, 44, 3407–3411 CrossRef CAS.
- (a) D. Cahard and V. Bizet, Chem. Soc. Rev., 2014, 43, 135 RSC; (b) V. Bizet and D. Cahard, Chimia, 2014, 68, 378 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra02224k |
| This journal is © The Royal Society of Chemistry 2018 |