Open Access Article
Open Access ArticleThe influence of substituent field and resonance effects on the ease of N-heterocyclic carbene formation from imidazolium rings†
Wojciech P. Oziminski *ab and
Christopher A. Ramsden
*ab and
Christopher A. Ramsden *c
*c
aNational Medicines Institute, 30/34 Chełmska Street, 00-725 Warsaw, Poland
bFaculty of Pharmacy, Medical University of Warsaw, 1 Banacha Street, 02-097 Warsaw, Poland
cSchool of Chemical and Physical Sciences, Lennard-Jones Laboratories, Keele University, Staffordshire ST5 5BG, UK. E-mail: c.a.ramsden@keele.ac.uk; wojozim@gmail.com
First published on 18th April 2018
Abstract
Using a set of twelve selected substituents, the influence of substituent properties on the ease of deprotonation of imidazolium cations and mesoionic imidazolium-4-olates measured by the CREF index has been investigated. Significant correlations between CREF values and the Swain and Lupton field (F) and resonance (R) substituent constants have been found. In all cases the field effect has the greatest influence but resonance effects are also significant.
Introduction
We have demonstrated that the deprotonation energy (ZPE corrected) of N-heterocyclic carbene (NHC) precursors, formulated as an index, provides a convenient overview of the relative ease of formation of a wide range of normal (nNHC), abnormal (aNHC) and remote (rNHC) neutral and anionic NHCs.1,2 This index, which we describe as the CREF (Carbene Relative Energy of Formation) index, is easily calculated using modern DFT methods. The results direct attention to properties of novel or unexplored molecules of potential interest, including their potential σ-bonding strength as ligands.In addition, we have observed, in accord with the Hammond postulate,3 that there is good correlation between CREF indexes and experimental rate constants for deprotonation or H–D exchange for a range of heterocycles for which kinetic data is available.
Our previous studies have been largely restricted to the influence of ring heteroatoms (N, O and S) on NHC formation. We now report a study of the influence of ring substituents.
Results and discussion
To explore the influence of ring substituents on NHC formation, we selected a set of eleven common substituents that represents a broad spectrum of sigma- and pi-electron-donor and electron-acceptor characteristics. The unsubstituted molecules were also included for reference. These substituents are shown in Table 1 together with their Swain and Lupton field (F) and resonance (R) constants.4 Each parameter shows good variation (F 0.0 to +0.65 and R −0.73 to +0.42), and there is low correlation between the two (r 0.56).| Substituent | F | R |
|---|---|---|
| NHMe | 0.03 | −0.73 |
| OMe | 0.29 | −0.56 |
| F | 0.45 | −0.39 |
| Cl | 0.42 | −0.19 |
| Me | 0.01 | −0.18 |
| cycHex | 0.03 | −0.18 |
| OCF3 | 0.39 | −0.04 |
| H | 0.00 | 0.00 |
| NO2 | 0.65 | 0.13 |
| CN | 0.51 | 0.15 |
| CF3 | 0.38 | 0.16 |
| NO | 0.49 | 0.42 |
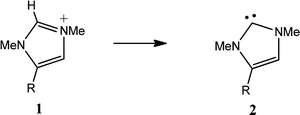
Initially we studied the influence of 4-substituents on the formation of the NHCs 2 by deprotonation of the imidazolium cations 1. The CREF values for these transformations are shown in Table 2 and multiple regression analysis shows a statistically significant relationship (eqn (1), Table 2A) between CREF values and the Swain and Lupton constants. As might be expected, since deprotonation primarily involves heterolytic cleavage of a σ bond, the larger effect is the field effect, but the resonance effect is also significant. Neither parameter alone (F nor R) gives a significant correlation. Using eqn (1) the CREF values of most common organic substituents can be estimated using published F and R values.
The observed substituent effects depend on the relative effects of substituents on reactant and product. Electron-withdrawing substituents reduce density around neighbouring atomic nuclei, exposing greater nuclear charge, and thus lower σ-orbital energies in both reactant and product.
| A | B | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CREF = 0.416(±0.002) − 0.038(±0.004)F − 0.021(±0.003)R (1), n = 12, r = 0.98, s = 0.003, F = 134.0, p < 0.0001 | CREF = 0.418(±0.004) − 0.069(±0.009)F − 0.028(±0.006)R (2), n = 12, r = 0.97, s = 0.006, F = 69.1, p < 0.0001 | ||||||||
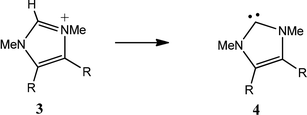
| Subst. R | Precursor 1 E + ZPEa | Product 2 E + ZPEa | CREF | Subst. R | Precursor 3 E + ZPEa | Product 4 E + ZPEa | CREF | ||
| a Hartrees. | |||||||||
| a | NHMe | −399.8209 | −399.3944 | 0.427 | a | NHMe | −494.4616 | −494.0342 | 0.427 |
| b | OMe | −419.6950 | −419.2753 | 0.420 | b | OMe | −534.2104 | −533.7884 | 0.422 |
| c | F | −404.4263 | −404.0218 | 0.405 | c | F | −503.6798 | −503.2840 | 0.396 |
| d | Cl | −764.7876 | −764.3815 | 0.406 | d | Cl | −1224.4070 | −1224.0071 | 0.400 |
| e | Me | −344.4758 | −344.0567 | 0.419 | e | Me | −383.7819 | −383.3579 | 0.424 |
| f | cycHex | −539.7656 | −539.3424 | 0.423 | f | cycHex | −774.3580 | −773.9273 | 0.431 |
| g | OCF3 | −717.5314 | −717.1286 | 0.403 | g | OCF3 | −1129.8927 | −1129.4988 | 0.394 |
| h | H | −305.1679 | −304.7548 | 0.413 | h | H | −305.1679 | −304.7548 | 0.413 |
| i | NO2 | −509.6969 | −509.3096 | 0.387 | i | NO2 | −714.2134 | −713.8449 | 0.369 |
| j | CN | −397.4147 | −397.0229 | 0.392 | j | CN | −489.6589 | −489.2844 | 0.374 |
| k | CF3 | −642.2949 | −641.8977 | 0.397 | k | CF3 | −979.4124 | −979.0276 | 0.385 |
| l | NO | −434.4790 | −434.0888 | 0.390 | l | NO | −563.7831 | −563.4090 | 0.374 |
Since deprotonation effectively involves the transfer of one electron from a hydrogen 1s environment to a carbon σ environment, the lowering of σ energy levels might be expected to not only polarise the C–H bond in the precursor but also stabilise the product, which gains an extra sp2 σ electron. This is consistent with electron-withdrawing substituents, e.g., NO2, CN, CF3, lowering CREF values. Other effects may contribute; substituents that are π donors, e.g., NHMe, OMe, may stabilise the cationic precursors 1 relative to the products 2 contributing to higher CREF values. The origins of the electronic effects of substituents may be complex but eqn (1) conveniently summarises the net effect and there are both σ and π contributions.
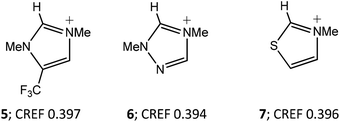
Fig. 1 illustrates the effect of three structural changes (cations 5–7) on the CREF index of the imidazolium cation 1h (CREF 0.413). A 4-trifluoromethyl substituent lowers the value by 0.016 units, which is comparable to the effect of 4-aza substitution. Cations 5 and 6 both have acidity comparable to the 1,3-thiazolium cation 7 (Fig. 1). The effect of a 4-nitro substituent (1i, Table 2) is even larger (Δ CREF 0.026 units). We have previously estimated that a reduction of the index by 0.01 units leads to a reduction of aqueous pKa of 2.4.2 On this basis, the estimated pKa of 16.8 for the 4-nitroimidazolium ring is comparable to that of the 1,3-oxazolium cation (pKa 16.9).5 The associated weakening of the C(2)–H bond in 4-nitroimidazolium rings is consistent with their formation of hydrogen bonds with chloride ions.6
The effect of a second substituent is cumulative. An equally significant correlation (eqn (2), Table 2B) was obtained for deprotonation of the disubstituted derivatives 3 giving the NHCs 4. Relative to eqn (1), the coefficients for F and R in eqn (2) are increased by 1.82 and 1.40, respectively. A number of 4,5-disubstituted imidazolium salts with electron-withdrawing substituents have been investigated as potential energetic ionic liquids.7 Based on the calculated CREF values, the dicyano derivative 3j (CREF 0.374) can be predicted to have pKa ∼ 13.6, and to have C–H acidity comparable to 1,4-dialkyltetrazolium salts (CREF 0.372).8–10
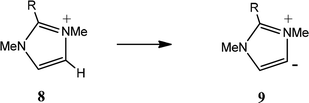
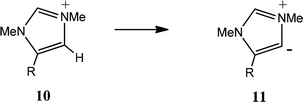
Similar statistically significant correlations between CREF index values and the Swain and Lupton constants were found for the formation the substituted aNHCs 9 (Table 3A) and 11 (Table 3B). For the deprotonation 8 → 9 the contributions of F and R are similar to those for the formation of the nNHCs 2 (Table 2A). This is consistent with the fact that the relationships between substituent location and carbene centre are similar. For the deprotonation 10 → 11 the contribution of F is greater and that of R is slightly smaller; in this case the substituents are adjacent to the carbene centre.
| A | B | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CREF = 0.445(±0.002) − 0.042(±0.006)F − 0.022(±0.004)R (3), n = 12, r = 0.97, s = 0.004, F = 72.8, p < 0.0001 | CREF = 0.445(±0.002) − 0.048(±0.005)F − 0.016(±0.003)R (4), n = 12, r = 0.98, s = 0.003, F = 106.0, p < 0.0001 | ||||||||
| Subst. R | Precursor 8 E + ZPEa | Product 9 E + ZPEa | CREF | Subst. R | Precursor 10 E + ZPEa | Product 11 E + ZPEa | CREF | ||
| a Hartrees. | |||||||||
| a | NHMe | −399.8211 | −399.3675 | 0.454 | a | NHMe | −399.8209 | −399.3696 | 0.451 |
| b | OMe | −419.6930 | −419.2447 | 0.448 | b | OMe | −419.6950 | −419.2521 | 0.443 |
| c | F | −404.4274 | −403.9925 | 0.435 | c | F | −404.4263 | −403.9974 | 0.429 |
| d | Cl | −764.7879 | −764.3522 | 0.436 | d | Cl | −764.7876 | −764.3553 | 0.432 |
| e | Me | −344.4771 | −344.0280 | 0.449 | e | Me | −344.4758 | −344.0276 | 0.448 |
| f | cycHex | −539.7674 | −539.3132 | 0.454 | f | cycHex | −539.7656 | −539.3137 | 0.452 |
| g | OCF3 | −717.5305 | −717.0970 | 0.434 | g | OCF3 | −717.5314 | −717.1054 | 0.426 |
| h | H | −305.1679 | −304.7258 | 0.442 | h | H | −305.1679 | −304.7258 | 0.442 |
| i | NO2 | −509.6897 | −509.2772 | 0.413 | i | NO2 | −509.6969 | −509.2861 | 0.411 |
| j | CN | −397.4117 | −396.9924 | 0.419 | j | CN | −397.4147 | −396.9984 | 0.416 |
| k | CF3 | −642.2870 | −641.8610 | 0.426 | k | CF3 | −642.2949 | −641.8727 | 0.422 |
| l | NO | −434.4773 | −434.0640 | 0.413 | l | NO | −434.4790 | −434.0628 | 0.416 |
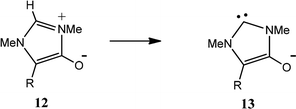
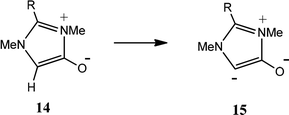
We have also investigated the effect of substituents on anionic NHC formation (Table 4). The CREF index values for anionic NHCs are higher, not least because the deprotonation process involves charge separation. Again, significant correlations are observed. For the formation of the nNHCs (12 → 13) (Table 4A), the correlation between CREF values and the electronic parameters F and R (eqn (5)) is very similar to that for the deprotonations 1 → 2 (Table 2A) and 8 → 9 (Table 3A). For formation of the anionic aNHCs (14 → 15) (Table 4B), the contribution of the field effect (F) (eqn (6)) is appreciably larger. The reason for this enhanced field effect is not clear, but it may be due to the association of position 4 with particularly high electron density.
| A | B | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CREF = 0.573(±0.003) − 0.040(±0.008)F − 0.023(±0.005)R (5), n = 12, r = 0.95, s = 0.005, F = 44.0, p < 0.0001 | CREF = 0.615(±0.004) − 0.069(±0.011)F − 0.026(±0.008)R (6), n = 12, r = 0.95, s = 0.007, F = 46.3, p < 0.0001 | ||||||||
| Subst. R | Precursor 12 E + ZPEa | Product 13 E + ZPEa | CREF | Subst. R | Precursor 14 E + ZPEa | Product 15 E + ZPEa | CREF | ||
| a Hartrees. | |||||||||
| a | NHMe | −474.6740 | −474.0966 | 0.577 | a | NHMe | −474.6720 | −474.0542 | 0.618 |
| b | OMe | −494.5473 | −493.9711 | 0.576 | b | OMe | −494.5498 | −493.9350 | 0.615 |
| c | F | −479.2917 | −478.7231 | 0.569 | c | F | −479.2966 | −478.6949 | 0.602 |
| d | Cl | −839.6561 | −839.0907 | 0.565 | d | Cl | −839.6556 | −839.0623 | 0.593 |
| e | Me | −419.3327 | −418.7548 | 0.578 | e | Me | −419.3331 | −418.7112 | 0.622 |
| f | cycHex | −614.6214 | −614.0457 | 0.576 | f | cycHex | −614.6186 | −613.9984 | 0.620 |
| g | OCF3 | −792.4036 | −791.8447 | 0.559 | g | OCF3 | −792.4013 | −791.8160 | 0.585 |
| h | H | −380.0312 | −379.4554 | 0.576 | h | H | −380.0312 | −379.4121 | 0.619 |
| i | NO2 | −584.5940 | −584.0559 | 0.538 | i | NO2 | −584.5863 | −584.0241 | 0.562 |
| j | CN | −472.3062 | −471.7592 | 0.547 | j | CN | −472.2981 | −471.7166 | 0.582 |
| k | CF3 | −717.1766 | −716.6242 | 0.552 | k | CF3 | −717.1658 | −716.5785 | 0.587 |
| l | NO | −509.3800 | −508.8396 | 0.540 | l | NO | −509.3748 | −508.8135 | 0.561 |
Computational details
Calculations were performed using the Gaussian 09 program,11 and the hybrid B3LYP functional,12,13 accompanied by the 6-311++G(d,p) basis set,14 was used. All geometry optimizations were followed by frequency calculations to ensure that the stationary points obtained were true minima on the potential energy surface and to calculate the zero-point vibrational corrections (ZPE) to energy. The CREF index was calculated as the (ZPE-corrected) energy difference the between protonated precursor and the carbene (obtained via C–H bond heterolytic cleavage).The multiple regression relationships and associated statistics based on the ordinary least squares method were determined using online software.15 The meaning of the statistical parameters are the following: n = sample size, r = multiple regression correlation coefficient, s = residual standard deviation, F = Fisher test value, p = p-value.
Conclusions
Statistically significant correlations have been found between the CREF index, which is a measure of C–H acidity, and electronic substituent constants for imidazolium cations and mesoionic imidazolium-4-olates. Both σ and π contributions to the acidity are observed. Derivatives disubstituted with electron-withdrawing groups (e.g., CF3, CN, NO2) are predicted to have acidities comparable to 1,3-dialkyltetrazolium cations,9 but without the instability associated with rapid loss of nitrogen.16The CREF index is a measure of the strength of C–H σ bonds in heteroaromatic rings, and, by analogy, the strength of C–C σ bonds (relevant to decarboxylation) and C-metal σ bonds, for example when NHCs act as metal ligands. In the latter case, there is no measure of C-metal π bonding (or steric effects). In the context of ligand binding, the effectiveness of metal complexes of the imidazole-ylidenes 4 as catalysts has been found to vary with ring substituents.17 This has been attributed to possible variation in π bonding between ligand and metal. This may be the case, but the correlations described in Table 2 show that π effects also influence σ bond strength and thus catalytic efficiency.
In 1966 Olofson and Landesberg concluded, justifiably, that the use of LCAO-MO methods to predict H/D exchange in heterocyclic cations was futile.18 The DFT approach that we have explored previously1,2 and extended in this study seems more promising. Experimentally determined physical properties of heterocycles are limited and there are gaps in the literature.19 It is hoped that the DFT-based approach described above will be helpful in understanding variations in physical properties of heteroaromatic rings and augment experimental data.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Computational Grant G36-9 from the Interdisciplinary Centre for Mathematical and Computational Modelling at Warsaw University (ICM UW) is gratefully acknowledged.Notes and references
- C. A. Ramsden and W. P. Oziminski, J. Org. Chem., 2016, 81, 10295 CrossRef CAS PubMed.
- C. A. Ramsden and W. P. Oziminski, J. Org. Chem., 2017, 82, 12485 CrossRef CAS PubMed.
- G. S. Hammond, J. Am. Chem. Soc., 1955, 77, 334 CrossRef CAS.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165 CrossRef CAS.
- T. L. Amyes, S. T. Diver, J. P. Richard, F. M. Rivas and K. Toth, J. Am. Chem. Soc., 2004, 126, 4366 CrossRef CAS PubMed.
- H. Ihm, S. Yun, H. G. Kim, J. K. Kim and K. S. Kim, Org. Lett., 2002, 4, 2897 CrossRef CAS PubMed.
- A. R. Katritzky, H. Yang, D. Zhang, K. Kirichenko, M. Smiglak, J. D. Holbrey, W. M. Reichert and R. D. Rogers, New J. Chem., 2006, 30, 349 RSC.
- R. A. Olofson, W. R. Thompson and J. S. Michelman, J. Am. Chem. Soc., 1964, 86, 1865 CrossRef CAS.
- A. C. Rochat and R. A. Olofson, Tetrahedron Lett., 1969, 10, 3377 CrossRef.
- G. D. Frey, K. Öfele, H. G. Krist, E. Herdtweck and W. A. Herrmann, Inorg. Chim. Acta, 2006, 359, 2622 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623 CrossRef CAS.
- R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, J. Chem. Phys., 1980, 72, 650 CrossRef CAS.
- P. Wessa, Multiple Regression (v1.0.48) in Free Statistics Software (v1.2.1), Office for Research Development and Education, 2017, https://www.wessa.net/rwasp_multipleregression.wasp Search PubMed.
- D. M. Zimmerman and R. A. Olofson, Tetrahedron Lett., 1970, 11, 3453 CrossRef.
- D. M. Khramov, E. L. Rosen, J. A. V. Er, P. D. Vu, V. M. Lynch and C. W. Bielawski, Tetrahedron, 2008, 64, 6853 CrossRef CAS.
- R. A. Olofson and J. M. Landesberg, J. Am. Chem. Soc., 1966, 88, 4263 CrossRef CAS.
- A. R. Katritzky, C. A. Ramsden, J. A. Joule and V. V. Zhdankin, Handbook of Heterocyclic Chemistry, 3rd edn, Elsevier, Oxford, 2010 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra02526f |
| This journal is © The Royal Society of Chemistry 2018 |